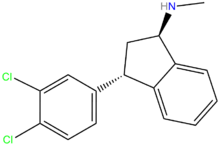
Indatraline
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
86939-10-8 |
| PubChem (CID) | 126280 |
| ChemSpider |
16736639 |
| UNII |
4U40Y96J1Z |
| ChEMBL |
CHEMBL146332 |
| Chemical and physical data | |
| Formula | C16H15Cl2N |
| Molar mass | 292.2072 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Indatraline (Lu 19-005) is a non-selective monoamine transporter inhibitor that has been shown to block the reuptake of dopamine, norepinephrine, and serotonin with effects similar to those of cocaine. However, the effects have been shown to have slower onset and longer duration than cocaine, suggesting that the compound may, along with similar compounds, be used for treatment of cocaine addiction.[1] Apparently, Lu 19-005 can be used to block the action of methamphetamine and MDMA.[2]
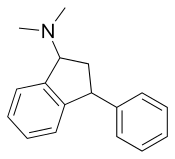
Compare Indatraline with tametraline since they are directly homologous. There are some differences though. Superposition should make it possible to see that there is at least a relationship between the pharmacophore of indatraline and various phenyltropanes. More recently, additional work has been done also.[3]
Trans
If indatraline is N-alkylated it is possible to slow the onset of action, if the choice of alkyl group is sufficiently bulky, so that it is not until N-demethylation occurs that the molecules become really active.[3] N-methyl indatraline has a much longer duration than indatraline, because norindatraline is inactive whereas demethylating N-methyl-indatraline does not terminate the actions of the parent compound. Most drug addiction programs are of the opinion that a "slow onset, long duration" DRI is less likely to be abused (c.f. Volkow and others). The N-methyl products are clearly not just inactive prodrugs though.
Measurement of ambulation was recorded in 10-minute bins. Whereas for cocaine, locomotor activity (LMA) was already apparent at 10 min, at least 20 minutes were needed before the NMe2 analogs even started to increase locomotor activity. Very high doses actually meant less ambulation. The reason for this was increased stereotypy. It is apparent from the table that larger N-groups result in increased DAT selectivity relative to the SERT and NET. Although it is not necessary to enter into the details, the authors actually wanted a SNDRI.
Racemic
N-Demethyl analogs situated on the right-side of the arrow, assuming that this is the way they are metabolized in vivo.
[125I]RTI-55 used as the binding ligand at all 3 transporters.
| Racemic (trans) 3',4'-Dichloro Indamines. Ki and IC50 (nM) | ||||||||
| Stereo | R1 | R2 | DAT | DA | SERT | 5-HT | NET | NE |
| Rac | Me | Me | 19 → 27 | 200 → 23 | 0.33 → 5.0 | 65 → 4.8 | 95 → 22 | 34 → 15 |
| Rac | Et | Me | 39 → 8.1 | 270 → 61 | 16 → 2.0 | 24 → 13 | 250 → 48 | 150 → 28 |
| Rac | Pr | Me | 250 → 220 | 340 → 53 | 91 → 130 | 190 → 480 | 400 → 55 | 640 → 130 |
| Rac | Pri | Me | 180 → 32 | 190 → 51 | 44 → 93 | 1500 → 240 | 260 → 110 | 420 → 75 |
| Rac | But | Me | 890 → 130 | 2700 → 370 | 4700 → 740 | >10μM → 3100 | 440 → 150 | 1000 → 310 |
| Rac | Bn | Me | 120 → 80 | 550 → 380 | 180 → >10μM | 3700 → >10μM | 2800 → 460 | 1600 → 2600 |
Single enantiomers
| Trans 3',4'-Dichloro indamines. Ki and IC50 (nM) | ||||||||
| Stereo | R1 | R2 | DAT | DA | SERT | 5-HT | NET | NE |
| (±)-trans | Me | Me | 19 | 200 | 0.33 | 65 | 95 | 34 |
| (+)-trans | Me | Me | 8.7 | 120 | 0.06 | 6.3 | 52 | 21 |
| (–)-trans | Me | Me | 38 | 650 | 3.6 | 130 | 130 | 130 |
The eudysmic ratio between the two enantiomers of N-methyl-indatraline is not as high as was reported in the case of tametraline. The toxicity of the R,S isomer is less than for the S,R isomer.
Cis
| Cis 3',4'-Dichloro indamines. Ki and IC50 (nM) | ||||||||
| R1 | R2 | DAT | DA | SERT | 5-HT | NET | NE | |
| Me | Me | 63 → 290 | 550 → 500 | 2.4 → 38 | 5.5 → 4.0 | 150 → 600 | 86 → 130 | |
| Et | Me | 660 → 140 | 530 → 1000 | 13 → 4.7 | 72 → 19 | 2400 → ? | 4400 → 170 | |
| Pr | Me | 1200 → 280 | 1000 → 620 | 200 → 500 | 810 → 1300 | 2000 → 540 | 540 → 630 | |
| Pri | Me | 680 → 450 | 960 → 1400 | 73 → 130 | 1800 → 300 | 650 → 3500 | 1800 → 1500 | |
| But | Me | 2800 → 650 | >10μM → 1500 | >10μM → 1700 | ? → >10μM | 2200 → 1100 | >10μM → 1800 | |
| Bn | Me | >10μM → 1100 | ? → 5300 | 300 → 2100 | 700 → >10μM | >10μM → 4800 | >10μM → >10μM | |
Chemistry
Two main routes have been reported. The first route shown is the original one reported by Bøgesø and co-workers.[4]
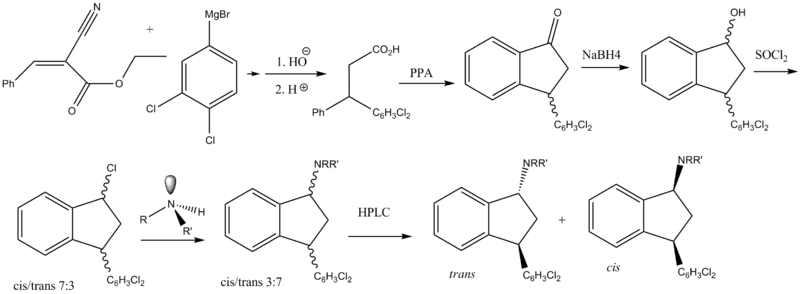
the other had been adapted to scale-up:[5]

Although a new method of making indatraline was recently published,[6] there is obviously more than one way of making this compound.
See for example: 6525206
Unfortunately for the indanone intermediates a method is not available for direct reduction of the imine or oxime. It is reported that the wrong diastereomers are formed (cis) whereas the trans isomers are the ones that are needed. This frustrates the synthesis since an extra step has to be inserted. First the ketones are reduced to get mostly cis alcohols, which are then converted to the corresponding mesylates conserving stereochemistry. These can then be reacted with e.g. N-methylbenzylamine, effecting a Walden inversion (SN2). Final removal of the benzyl affords product, although it is racemic.
See also
References
- ↑ Negus, S. S.; Brandt, M. R.; Mello, N. K. (1999). "Effects of the long-acting monoamine reuptake inhibitor indatraline on cocaine self-administration in rhesus monkeys". The Journal of Pharmacology and Experimental Therapeutics. 291 (1): 60–69. PMID 10490887.
- ↑ Rothman, R. B.; Partilla, J. S.; Baumann, M. H.; Dersch, C. M.; Carroll, F. I.; Rice, K. C. (2000). "Neurochemical neutralization of methamphetamine with high-affinity nonselective inhibitors of biogenic amine transporters: a pharmacological strategy for treating stimulant abuse". Synapse. 35 (3): 222–7. doi:10.1002/(SICI)1098-2396(20000301)35:3<222::AID-SYN7>3.0.CO;2-K. PMID 10657029.
- 1 2 Gardner, E.; Liu, X.; Paredes, W.; Giordano, A.; Spector, J.; Lepore, M.; Wu, K.; Froimowitz, M. (2006). "A slow-onset, long-duration indanamine monoamine reuptake inhibitor as a potential maintenance pharmacotherapy for psychostimulant abuse: effects in laboratory rat models relating to addiction". Neuropharmacology. 51 (5): 993–1003. doi:10.1016/j.neuropharm.2006.06.009. PMID 16901516.
- ↑ Bøgesø, K. P.; Christensen, A. V.; Hyttel, J.; Liljefors, T. (1985). "3-Phenyl-1-indanamines. Potential antidepressant activity and potent inhibition of dopamine, norepinephrine, and serotonin uptake". Journal of Medicinal Chemistry. 28 (12): 1817–1828. doi:10.1021/jm00150a012. PMID 2999402.
- ↑ Froimowitz, M.; Wu, K. M.; Moussa, A.; Haidar, R. M.; Jurayj, J.; George, C.; Gardner, E. L. (2000). "Slow-onset, long-duration 3-(3',4'-dichlorophenyl)-1-indanamine monoamine reuptake blockers as potential medications to treat cocaine abuse". Journal of Medicinal Chemistry. 43 (26): 4981–4992. doi:10.1021/jm000201d. PMID 11150168.
- ↑ Silva Lf, J.; Siqueira, F.; Pedrozo, E.; Vieira, F.; Doriguetto, A. (2007). "Iodine(III)-promoted ring contraction of 1,2-dihydronaphthalenes: a diastereoselective total synthesis of (+/-)-indatraline". Organic Letters. 9 (8): 1433–1436. doi:10.1021/ol070027o. PMID 17371034.
- ↑ Barltrop, J. A.; Acheson, R. M.; Philpott, P. G.; MacPhee, K. E.; Hunt, J. S. (1956). "570. Compounds of potential pharmacological interest. Part IV. Aryl and alkyl derivatives of 1-aminoindane". Journal of the Chemical Society (Resumed): 2928. doi:10.1039/JR9560002928.