Mephentermine
 | |
| Clinical data | |
|---|---|
| Routes of administration | IM/IV |
| ATC code | C01CA11 (WHO) |
| Pharmacokinetic data | |
| Metabolism | Rapidly demethylated in the body followed by hydroxylation. |
| Excretion | Via urine (as unchanged and metabolites); more rapid in acidic urine. |
| Identifiers | |
| |
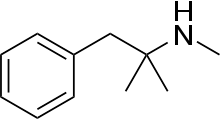
| Synonyms | methyl(2-methyl-1-phenylpropan-2-yl)amine |
| CAS Number |
100-92-5 |
| PubChem (CID) | 3677 |
| IUPHAR/BPS | 7222 |
| DrugBank |
DB01365 |
| ChemSpider |
3549 |
| UNII |
TEZ91L71V4 |
| KEGG |
D08180 |
| ChEMBL |
CHEMBL1201234 |
| ECHA InfoCard | 100.002.638 |
| Chemical and physical data | |
| Formula | C11H17N |
| Molar mass | 163.259 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Mephentermine is a cardiac stimulant. It was formerly used in Wyamine nasal decongestant inhalers and before that as a stimulant in psychiatry.
It has been used as a treatment for low blood pressure.[1]
ATC Classification: C01CA11 - mephentermine ; Belongs to the class of adrenergic and dopaminergic cardiac stimulants excluding glycosides. Used in the treatment of heart failure.
Mechanism of Action
Mephentermine appears to act by indirect stimulation of β-adrenergic receptors causing the release of norepinephrine from its storage sites. It has a positive inotropic effect on the myocardium. AV conduction and refractory period of AV node is shortened with an increase in ventricular conduction velocity. It dilates arteries and arterioles in the skeletal muscle and mesenteric vascular beds, leading to an increase in venous return.
Onset: 5–15 minutes (IM), immediate (IV).
Duration: 4 hr (IM), 30 minutes (IV).
Indication & Dosage
Maintenance of blood pressure in hypotensive states Adult: 30–45 mg as a single dose, repeated as necessary or followed by IV infusion of 0.1% mephentermine in 5% dextrose, rate and duration of administration will depend on patient's response.
Hypotension secondary to spinal anaesthesia in obstetric patients Adult: 15 mg as a single dose, repeat if needed.Maximum dose 30 mg.
Caution
Contraindications
Low blood pressure caused by phenothiazines. Hypertension. Pheochromocytoma.
Special Precautions
Patient on MAOIs. For shock due to loss of blood or fluid, give fluid replacement therapy primarily, CVS disease, hypertension, hyperthyroidism, chronic illnesses, lactation, pregnancy.
Adverse Drug Reactions
Drowsiness, incoherence, hallucinations, convulsions, slow heart rate (Reflex Bradycardia). Fear, anxiety, restlessness, tremor, insomnia, confusion, irritability, and psychosis. Nausea, vomiting, reduced appetite, urinary retention, dyspnea, weakness.
Potentially Fatal: AV block, CNS stimulation. Cerebral hemorrhage and pulmonary edema, ventricular arrhythmias.
Drug Interactions
Antagonizes effect of agents that lower blood pressure. Severe hypertension with MAOIs and possibly tricyclic antidepressants. Additive vasoconstricting effects with ergot alkaloids, oxytocin.
Potentially Fatal: Risk of abnormal heart rhythm in people undergoing anesthesia with cyclopropane and halothane.