Dichloropane
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number |
146725-34-0 |
| PubChem (CID) | 127024 |
| ChemSpider |
112783 |
| Chemical and physical data | |
| Formula | C15H17Cl2NO2 |
| Molar mass | 314.207 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
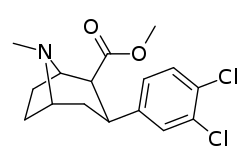
Dichloropane ((−)-2β-Carbomethoxy-3β-(3,4-dichlorophenyl)tropane, RTI-111, O-401) is a stimulant of the phenyltropane class that acts as a serotonin–norepinephrine–dopamine reuptake inhibitor (SNDRI) with IC50 values of 3.13, 0.79 and 18 nM, respectively.[1] In animal studies, dichloropane had a slower onset and longer duration of action compared to cocaine.[2][3]
Methylecgonidine as the direct precursor to this compound.[4]
Trans -CO2Me group
The thermodynamic isomer with a trans -CO2Me group is still active. This isomer was used by Neurosearch to make three different phenyltropanes which were tested in clinical trials.
- Tesofensine
- Brasofensine
- NS-2359 (GSK-372,475)
See also
References
- ↑ Carroll, F. Ivy; Blough, Bruce E.; Nie, Zhe; Kuhar, Michael J.; Howell, Leonard L.; Navarro, Hernan A. (21 April 2005). "Synthesis and Monoamine Transporter Binding Properties of 3β-(3',4'-Disubstituted phenyl)tropane-2β-carboxylic Acid Methyl Esters". Journal of Medicinal Chemistry. 48 (8): 2767–2771. doi:10.1021/jm040185a. PMID 15828814.
- ↑ Ranaldi, Robert; Anderson, Karen G.; Carroll, F. Ivy; Woolverton, William L. (December 2000). "Reinforcing and discriminative stimulus effects of RTI 111, a 3-phenyltropane analog, in rhesus monkeys: interaction with methamphetamine". Psychopharmacology. 153 (1): 103–110. doi:10.1007/s002130000602. ISSN 1432-2072. PMID 11255920.
- ↑ Cook, Charles D.; Carroll, Ivy F.; Beardsley, Patrick M. (December 2001). "Cocaine-like discriminative stimulus effects of novel cocaine and 3-phenyltropane analogs in the rat". Psychopharmacology. 159 (1): 58–63. doi:10.1007/s002130100891. ISSN 1432-2072. PMID 11797070.
- ↑ Carroll, F. Ivy; Mascarella, S. Wayne; Kuzemko, Michael A.; Gao, Yigong; Abraham, Philip; Lewin, Anita H.; Boja, John W.; Kuhar, Michael J. (2 September 1994). "Synthesis, Ligand Binding, and QSAR (CoMFA and Classical) Study of 3β-(3'-Substituted phenyl)-, 3β-(4'-Substituted phenyl)-, and 3β-(3',4'-Disubstituted phenyl)tropane-2β-carboxylic Acid Methyl Esters". Journal of Medicinal Chemistry. 37 (18): 2865–2873. doi:10.1021/jm00044a007. PMID 8071935.
| 2-Carboxymethyl Esters | |
|---|---|
| (3,4-Disubstituted Phenyl)-tropanes | |
| Arylcarboxy | |
| Carboxyalkyl | |
| Acyl | |
| β,α Stereochemistry | |
| α,β Stereochemistry | |
| Heterocycles: 3-Substituted-isoxazol-5-yl | |
| Heterocycles: 3-Substituted-1,2,4-oxadiazole | |
| N-alkyl | |
| N-replaced (S,O,C) | |
| Irreversible | |
| Nortropanes (N-demethylated) | |
This article is issued from Wikipedia - version of the 9/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.