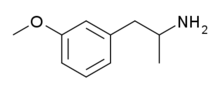
3-Methoxyamphetamine
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code | none |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
17862-85-0 |
| PubChem (CID) | 152234 |
| ChemSpider |
134180 |
| ChEMBL |
CHEMBL16247 |
| Chemical and physical data | |
| Formula | C10H15NO |
| Molar mass | 165.232 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
meta-Methoxyamphetamine[1] (MMA), also known as 3-methoxyamphetamine (3-MA), is a stimulant drug from the amphetamine family. It has similar effects in animal drug discrimination tests to the more widely known derivative 4-methoxyamphetamine (PMA),[2] although with a slightly different ratio of monoamine release, being a combined serotonin, dopamine, and norepinephrine releasing agent rather than a fairly selective serotonin releaser like PMA.[3][4] 3-Methoxyamphetamine has similarly appeared on the illicit market as a designer drug alternative to MDMA, although far more rarely than its infamous positional isomer.[5] It produces gepefrine, a cardiac stimulant, as one of its major metabolites.[6]
See also
- 2-Methoxyamphetamine (OMA)
- 3-Methylamphetamine (3-MA)
- 3-Fluoroamphetamine (3-FA)
- 3-Trifluoromethylamphetamine (Norfenfluramine)
- 3-Methoxy-4-methylamphetamine (MMA)
- 3-Methoxymethamphetamine (MMMA)
- 4-Ethoxyamphetamine (4-ETA)
References
- ↑ GB Patent 1527479 - ACID ADDITION SALTS OF D-(+)-1-(3-HYDROXYPHENYL)-2-AMINOPROPANE AND THEIR MANUFACTURE AND USE
- ↑ Glennon, RA; Young, R; Hauck, AE (1985). "Structure-activity studies on methoxy-substituted phenylisopropylamines using drug discrimination methodology". Pharmacology, Biochemistry, and Behavior. 22 (5): 723–9. doi:10.1016/0091-3057(85)90520-9. PMID 3839309.
- ↑ Tseng, LF; Menon, MK; Loh, HH (1976). "Comparative actions of monomethoxyamphetamines on the release and uptake of biogenic amines in brain tissue". The Journal of Pharmacology and Experimental Therapeutics. 197 (2): 263–71. PMID 1271280.
- ↑ Menon, MK; Tseng, LF; Loh, HH (1976). "Pharmacological evidence for the central serotonergic effects of monomethoxyamphetamines". The Journal of Pharmacology and Experimental Therapeutics. 197 (2): 272–9. PMID 946817.
- ↑ Dal Cason, TA (2001). "A re-examination of the mono-methoxy positional ring isomers of amphetamine, methamphetamine and phenyl-2-propanone". Forensic Science International. 119 (2): 168–94. doi:10.1016/S0379-0738(00)00425-4. PMID 11376983.
- ↑ Midha, KK; Cooper, JK; Bailey, K; Hubbard, JW (1981). "The metabolism of 3-methoxyamphetamine in dog, monkey and man". Xenobiotica. 11 (2): 137–46. doi:10.3109/00498258109045284. PMID 6894510.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.