Tryptophan
 | |
| Names | |
|---|---|
| IUPAC name
Tryptophan or (2S)-2-amino-3-(1H-indol-3-yl)propanoic acid | |
| Other names
2-Amino-3-(1H-indol-3-yl)propanoic acid | |
| Identifiers | |
| 73-22-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:27897 |
| ChEMBL | ChEMBL54976 |
| ChemSpider | 6066 |
| DrugBank | DB00150 |
| ECHA InfoCard | 100.000.723 |
| 717 | |
| KEGG | D00020 |
| PubChem | 6305 |
| UNII | 8DUH1N11BX |
| |
| |
| Properties | |
| C11H12N2O2 | |
| Molar mass | 204.23 g·mol−1 |
| Soluble: 0.23 g/L at 0 °C, 11.4 g/L at 25 °C, | |
| Solubility | Soluble in hot alcohol, alkali hydroxides; insoluble in chloroform. |
| Acidity (pKa) | 2.38 (carboxyl), 9.39 (amino)[1] |
| Pharmacology | |
| N06AX02 (WHO) | |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tryptophan (abbreviated as Trp or W; encoded by the codon UGG) is an α-amino acid that is used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated –NH3+ form under biological conditions), an α-carboxylic acid group (which is in the deprotonated –COO− form under biological conditions), and a side chain indole, classifying it as a non-polar, aromatic amino acid. It is essential in humans, meaning the body cannot synthesize it and thus it must be obtained from the diet.
Tryptophan is also a precursor to the neurotransmitters serotonin and melatonin.[2]
Isolation
The isolation of tryptophan was first reported by Frederick Hopkins in 1901[3] through hydrolysis of casein. From 600 grams of crude casein one obtains 4-8 grams of tryptophan.[4]
Biosynthesis and industrial production
As an essential amino acid, tryptophan is not synthesized from more basic substances in humans and other animals, who must ingest tryptophan or tryptophan-containing proteins. Plants and microorganisms commonly synthesize tryptophan from shikimic acid or anthranilate[5] by the following process: anthranilate condenses with phosphoribosylpyrophosphate (PRPP), generating pyrophosphate as a by-product. The ring of the ribose moiety is opened and subjected to reductive decarboxylation, producing indole-3-glycerinephosphate; this, in turn, is transformed into indole. In the last step, tryptophan synthase catalyzes the formation of tryptophan from indole and the amino acid serine.
The industrial production of tryptophan is also biosynthetic and is based on the fermentation of serine and indole using either wild-type or genetically modified bacteria such as B. amyloliquefaciens, B. subtilis, C. glutamicum or E. coli. These strains carry either mutations that prevent the reuptake of aromatic amino acids or multiple/overexpressed trp operons. The conversion is catalyzed by the enzyme tryptophan synthase.[6][7][8]
Function

For many organisms (including humans), tryptophan is needed to prevent illness or death, but cannot be synthesized by the organism and must be ingested; in short, it is an essential amino acid. Amino acids, including tryptophan, act as building blocks in protein biosynthesis, and proteins are required to sustain life. In addition, tryptophan functions as a biochemical precursor for the following compounds (see also figure to the right):
- Serotonin (a neurotransmitter), synthesized via tryptophan hydroxylase.[9][10] Serotonin, in turn, can be converted to melatonin (a neurohormone), via N-acetyltransferase and 5-hydroxyindole-O-methyltransferase activities.[11]
- Niacin, also known as vitamin B3, is synthesized from tryptophan via kynurenine and quinolinic acids as key biosynthetic intermediates.[12]
- Auxins (a class of phytohormones) are synthesized from tryptophan.[13]
The disorder fructose malabsorption causes improper absorption of tryptophan in the intestine, reduced levels of tryptophan in the blood,[14] and depression.[15] Some studies did not find reduced tryptophan in cases of lactose maldigestion.[14]
In bacteria that synthesize tryptophan, high cellular levels of this amino acid activate a repressor protein, which binds to the trp operon.[16] Binding of this repressor to the tryptophan operon prevents transcription of downstream DNA that codes for the enzymes involved in the biosynthesis of tryptophan. So high levels of tryptophan prevent tryptophan synthesis through a negative feedback loop and, when the cell's tryptophan levels are reduced, transcription from the trp operon resumes. The genetic organisation of the trp operon thus permits tightly regulated and rapid responses to changes in the cell's internal and external tryptophan levels.
Dietary sources
Tryptophan is a routine constituent of most protein-based foods or dietary proteins. It is particularly plentiful in chocolate, oats, dried dates, milk, yogurt, cottage cheese, red meat, eggs, fish, poultry, sesame, chickpeas, almonds, sunflower seeds, pumpkin seeds, buckwheat, spirulina, and peanuts. Contrary to the popular belief[21][22][23] that turkey contains an abundance of tryptophan, the tryptophan content in turkey is typical of poultry.[24]
| Food | Tryptophan [g/100 g of food] |
Protein [g/100 g of food] |
Tryptophan/Protein [%] |
|---|---|---|---|
| egg white, dried | | | |
| spirulina, dried | | | |
| cod, atlantic, dried | | | |
| soybeans, raw | | | |
| cheese, Parmesan | | | |
| sesame seed | | | |
| cheese, cheddar | | | |
| sunflower seed | | | |
| pork, chop | | | |
| turkey | | | |
| chicken | | | |
| beef | | | |
| oats | | | |
| salmon | | | |
| lamb, chop | | | |
| perch, Atlantic | | | |
| chickpeas, raw | | | |
| egg | | | |
| wheat flour, white | | | |
| baking chocolate, unsweetened | | | |
| milk | | | |
| rice, white, medium-grain, cooked | | | |
| quinoa, uncooked | | | |
| quinoa, cooked | | | |
| potatoes, russet | | | |
| tamarind | | | |
| banana | | | |
Turkey meat and drowsiness
A common assertion in the US is that heavy consumption of turkey meat results in drowsiness, due to high levels of tryptophan contained in turkey.[21][23] However, the amount of tryptophan in turkey is comparable to that contained in other meats.[22][24] Drowsiness after eating may be caused by other foods eaten with the turkey, particularly carbohydrates.[26] It has been demonstrated in both animal[27] and human tests[28][29][30] that ingestion of a meal rich in carbohydrates triggers release of insulin. Insulin in turn stimulates the uptake of large neutral branched-chain amino acids (BCAA), but not tryptophan into muscle, increasing the ratio of tryptophan to BCAA in the blood stream. The resulting increased tryptophan ratio reduces competition at the large neutral amino acid transporter (which transports both BCAA and aromatic amino acids), resulting in more uptake of tryptophan across the blood–brain barrier into the cerebrospinal fluid (CSF).[31][32] Once in the CSF, tryptophan is converted into serotonin in the raphe nuclei by the normal enzymatic pathway.[27][29] The resultant serotonin is further metabolised into melatonin by the pineal gland.[11] Hence, this data suggests that "feast-induced drowsiness"—or postprandial somnolence—may be the result of a heavy meal rich in carbohydrates, which indirectly increases the production of sleep-promoting melatonin in the brain.[27][28][29][30]
Use as a dietary supplement
Tryptophan is sold over the counter in the United States, Canada, and the United Kingdom as a dietary supplement for use as an antidepressant, anxiolytic, and sleep aid. It is also marketed as a prescription drug in some European countries for the indication of major depression under various trade names.
Since tryptophan is converted into 5-hydroxytryptophan (5-HTP) which is subsequently converted into the neurotransmitter serotonin, it has been proposed that consumption of tryptophan or 5-HTP may therefore improve depression symptoms by increasing the level of serotonin in the brain. In 2001 a Cochrane Review of the effect of 5-HTP and tryptophan on depression was published. The authors included only studies of a high rigor and included both 5-HTP and tryptophan in their review because of the limited data on either. Of 108 studies of 5-HTP and tryptophan on depression published between 1966 and 2000, only two met the authors' quality standards for inclusion, totaling 64 study participants. The substances were more effective than placebo in the two studies included but the authors state that, "the evidence was of insufficient quality to be conclusive," and note, "because alternative antidepressants exist which have been proven to be effective and safe, the clinical usefulness of 5-HTP and tryptophan is limited at present."[33] The use of tryptophan as an adjunctive therapy in addition to standard treatment for mood and anxiety disorders is not supported by the scientific evidence.[34] Due to the lack of high quality studies and preliminary nature of studies showing effectiveness and the lack of adequate study on their safety, the use of tryptophan and 5-HTP is not highly recommended or thought to be clinically useful.[33][34]
There is evidence that blood tryptophan levels are unlikely to be altered by changing the diet,[35] but tryptophan is available in health food stores as a dietary supplement.[36] Consuming purified tryptophan increases brain serotonin whereas eating foods containing tryptophan does not.[37] This is because the transport system which brings tryptophan across the blood-brain barrier is also selective for the other amino acids which are contained in protein food sources.[38] High blood plasma levels of other large neutral amino acids prevent the plasma concentration of tryptophan from increasing brain concentration levels.[38]
Side effects
Potential side effects of tryptophan include nausea, diarrhea, drowsiness, lightheadedness, headache, dry mouth, blurred vision, sedation, euphoria, and nystagmus (involuntary eye movements).[39][40] Because tryptophan has not been thoroughly studied in a clinical setting, possible side effects and interactions with other drugs are not well known.[33]
Interactions
Tryptophan taken as a dietary supplement (such as in tablet form) has the potential to cause serotonin syndrome when combined with antidepressants of the MAOI or SSRI class or other strongly serotonergic drugs.[40]
Research
In 1912 Felix Ehrlich demonstrated that yeast attacks the natural amino acids essentially by splitting off carbon dioxide and replacing the amino group with hydroxyl. By this reaction, tryptophan gives rise to tryptophol.[41]
Tryptophan affects brain serotonin synthesis when given orally in a purified form and is used to modify serotonin levels for research in psychology.[37] Low brain serotonin is induced by administration of tryptophan-poor protein in a technique called 'acute tryptophan depletion'.[42] Studies using this method have evaluated the effect of serotonin on mood and social behavior, finding that serotonin reduces aggression and increases agreeableness.[43]
Fluorescence
Tryptophan is an important intrinsic fluorescent probe (amino acid), which can be used to estimate the nature of microenvironment of the tryptophan. Most of the intrinsic fluorescence emissions of a folded protein are due to excitation of tryptophan residues.
Safety
Eosinophilia–myalgia syndrome
There was a large outbreak of eosinophilia-myalgia syndrome (EMS) in the U.S. in 1989, with more than 1,500 cases reported to the CDC and at least 37 deaths. After preliminary investigation revealed that the outbreak was linked to intake of tryptophan, the U.S. Food and Drug Administration (FDA) banned most tryptophan from sale in the US in 1991, and other countries followed suit.[44]
Subsequent epidemiological studies suggested that EMS was linked to specific batches of L-tryptophan supplied by a single large Japanese manufacturer, Showa Denko.[44][45][46][47] It eventually became clear that recent batches of Showa Denko's L-tryptophan were contaminated by trace impurities, which were subsequently thought to be responsible for the 1989 EMS outbreak.[44][48][49] However, other evidence suggests that tryptophan itself may be a potentially major contributory factor in EMS.[50]
The FDA loosened its restrictions on sales and marketing of tryptophan in February 2001, but continued to limit the importation of tryptophan not intended for an exempted use until 2005.[44]
The fact that the Showa Denko facility used genetically engineered bacteria to produce the contaminated batches of L-tryptophan later found to have caused the outbreak of eosinophilia-myalgia syndrome has been cited as evidence of a need for "close monitoring of the chemical purity of biotechnology-derived products."[51] Those calling for purity monitoring have, in turn, been criticized as anti-GMO activists who overlook possible non-GMO causes of contamination and threaten the development of biotech.[52]
See also
- 5-HTP
- Acree-Rosenheim reaction
- Adamkiewicz reaction
- attenuator (genetics)
- dimethyltryptamine
- Hopkins Cole reaction
- serotonin
- tryptamine
References
- ↑ Dawson RM, et al. (1969). Data for Biochemical Research. Oxford: Clarendon Press. ISBN 0-19-855338-2.
- ↑ Slominski A, Semak I, Pisarchik A, Sweatman T, Szczesniewski A, Wortsman J (January 2002). "Conversion of L-tryptophan to serotonin and melatonin in human melanoma cells". FEBS Letters. 511 (1-3): 102–6. doi:10.1016/s0014-5793(01)03319-1. PMID 11821057.
- ↑ Hopkins FG, Cole SW (December 1901). "A contribution to the chemistry of proteids: Part I. A preliminary study of a hitherto undescribed product of tryptic digestion". The Journal of Physiology. 27 (4-5): 418–28. doi:10.1113/jphysiol.1901.sp000880. PMC 1540554
 . PMID 16992614.
. PMID 16992614. - ↑ Cox GJ, King H (1943). "L-Tryptophane". Org. Synth. 2: 612–616. doi:10.15227/orgsyn.010.0100.
- ↑ Radwanski ER, Last RL (July 1995). "Tryptophan biosynthesis and metabolism: biochemical and molecular genetics". The Plant Cell. 7 (7): 921–34. doi:10.1105/tpc.7.7.921. PMC 160888
 . PMID 7640526.
. PMID 7640526. - ↑ Ikeda M (2002). "Amino acid production processes". Advances in Biochemical Engineering/Biotechnology. Advances in Biochemical Engineering/Biotechnology. 79: 1–35. doi:10.1007/3-540-45989-8_1. ISBN 978-3-540-43383-5. PMID 12523387.
- ↑ Becker J, Wittmann C (August 2012). "Bio-based production of chemicals, materials and fuels -Corynebacterium glutamicum as versatile cell factory". Current Opinion in Biotechnology. 23 (4): 631–40. doi:10.1016/j.copbio.2011.11.012. PMID 22138494.
- ↑ Conrado RJ, Varner JD, DeLisa MP (October 2008). "Engineering the spatial organization of metabolic enzymes: mimicking nature's synergy". Current Opinion in Biotechnology. 19 (5): 492–9. doi:10.1016/j.copbio.2008.07.006. PMID 18725290.
- ↑ Fernstrom JD (April 1983). "Role of precursor availability in control of monoamine biosynthesis in brain". Physiological Reviews. 63 (2): 484–546. PMID 6132421.
- ↑ Schaechter JD, Wurtman RJ (November 1990). "Serotonin release varies with brain tryptophan levels" (PDF). Brain Research. 532 (1-2): 203–10. doi:10.1016/0006-8993(90)91761-5. PMID 1704290.
- 1 2 Wurtman RJ, Anton-Tay F (1969). "The mammalian pineal as a neuroendocrine transducer" (PDF). Recent Progress in Hormone Research. 25: 493–522. doi:10.1016/b978-0-12-571125-8.50014-4. PMID 4391290.
- ↑ Ikeda M, Tsuji H, Nakamura S, Ichiyama A, Nishizuka Y, Hayaishi O (March 1965). "Studies on the biosynthesis of nicotinamide adenine dinucleotide. II. A role of picolinic carboxylase in the biosynthesis of nicotinamide adenine dinucleotide from tryptophan in mammals". The Journal of Biological Chemistry. 240 (3): 1395–401. PMID 14284754.
- ↑ Palme K, Nagy F (April 2008). "A new gene for auxin synthesis". Cell. 133 (1): 31–2. doi:10.1016/j.cell.2008.03.014. PMID 18394986.
- 1 2 Ledochowski M, Widner B, Murr C, Sperner-Unterweger B, Fuchs D (April 2001). "Fructose malabsorption is associated with decreased plasma tryptophan". Scandinavian Journal of Gastroenterology. 36 (4): 367–71. doi:10.1080/003655201300051135. PMID 11336160.
- ↑ Ledochowski M, Sperner-Unterweger B, Widner B, Fuchs D (June 1998). "Fructose malabsorption is associated with early signs of mental depression". European Journal of Medical Research. 3 (6): 295–8. PMID 9620891.
- ↑ Gollnick P, Babitzke P, Antson A, Yanofsky C (2005). "Complexity in regulation of tryptophan biosynthesis in Bacillus subtilis". Annual Review of Genetics. 39: 47–68. doi:10.1146/annurev.genet.39.073003.093745. PMID 16285852.
- 1 2 3 4 5 6 7 8 9 Zhang LS, Davies SS (April 2016). "Microbial metabolism of dietary components to bioactive metabolites: opportunities for new therapeutic interventions". Genome Med. 8 (1): 46. doi:10.1186/s13073-016-0296-x. PMC 4840492
 . PMID 27102537.
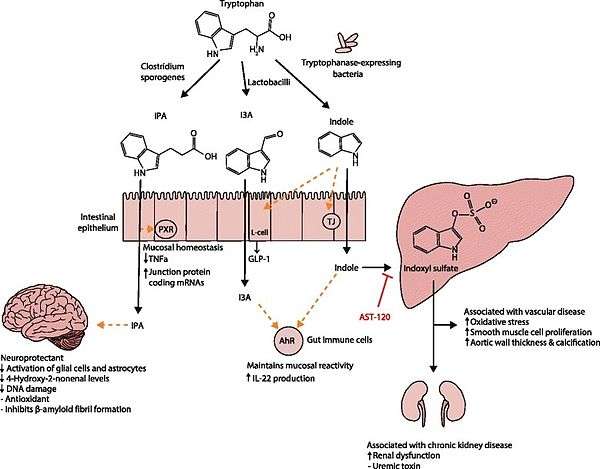
. PMID 27102537. Lactobacillus spp. convert tryptophan to indole-3-aldehyde (I3A) through unidentified enzymes [125]. Clostridium sporogenes convert tryptophan to IPA [6], likely via a tryptophan deaminase. ... IPA also potently scavenges hydroxyl radicals
Table 2: Microbial metabolites: their synthesis, mechanisms of action, and effects on health and disease
Figure 1: Molecular mechanisms of action of indole and its metabolites on host physiology and disease - ↑ Wikoff WR, Anfora AT, Liu J, Schultz PG, Lesley SA, Peters EC, Siuzdak G (2009). "Metabolomics analysis reveals large effects of gut microflora on mammalian blood metabolites". Proc. Natl. Acad. Sci. U.S.A. 106 (10): 3698–703. doi:10.1073/pnas.0812874106. PMC 2656143
 . PMID 19234110.
. PMID 19234110. Production of IPA was shown to be completely dependent on the presence of gut microflora and could be established by colonization with the bacterium Clostridium sporogenes.
IPA metabolism diagram - ↑ "3-Indolepropionic acid". Human Metabolome Database. University of Alberta. Retrieved 12 October 2015.
Indole-3-propionate (IPA), a deamination product of tryptophan formed by symbiotic bacteria in the gastrointestinal tract of mammals and birds. 3-Indolepropionic acid has been shown to prevent oxidative stress and death of primary neurons and neuroblastoma cells exposed to the amyloid beta-protein in the form of amyloid fibrils, one of the most prominent neuropathologic features of Alzheimer's disease. 3-Indolepropionic acid also shows a strong level of neuroprotection in two other paradigms of oxidative stress. (PMID 10419516 )
Origin: • Endogenous • Microbial - ↑ Chyan YJ, Poeggeler B, Omar RA, Chain DG, Frangione B, Ghiso J, Pappolla MA (1999). "Potent neuroprotective properties against the Alzheimer beta-amyloid by an endogenous melatonin-related indole structure, indole-3-propionic acid". J. Biol. Chem. 274 (31): 21937–42. doi:10.1074/jbc.274.31.21937. PMID 10419516.
[Indole-3-propionic acid (IPA)] has previously been identified in the plasma and cerebrospinal fluid of humans, but its functions are not known. ... In kinetic competition experiments using free radical-trapping agents, the capacity of IPA to scavenge hydroxyl radicals exceeded that of melatonin, an indoleamine considered to be the most potent naturally occurring scavenger of free radicals. In contrast with other antioxidants, IPA was not converted to reactive intermediates with pro-oxidant activity.
- 1 2 Helmenstine AM. "Does Eating Turkey Make You Sleepy?". About.com. Retrieved 2013-11-13.
- 1 2 Ballantyne C (2007-11-21). "Does Turkey Make You Sleepy?". Scientific American. Retrieved 2013-06-06.
- 1 2 McCue K. "Chemistry.org: Thanksgiving, Turkey, and Tryptophan". Archived from the original on 2007-04-04. Retrieved 2007-08-17.
- 1 2 3 Joanne Holden, Nutrient Data Laboratory, Agricultural Research Service. "USDA National Nutrient Database for Standard Reference, Release 22". United States Department of Agriculture. Retrieved 2009-11-29.
- ↑ Rambali B, Van Andel I, Schenk E, Wolterink G, van de Werken G, Stevenson H, Vleeming W (2002). "[The contribution of cocoa additive to cigarette smoking addiction]" (PDF). RIVM. The National Institute for Public Health and the Environment (Netherlands) (report 650270002/2002).
- ↑ "Food & mood. (neuroscience professor Richard Wurtman) (Interview)". Nutrition Action Healthletter. HighBeam Research. September 1992.
- 1 2 3 Fernstrom JD, Wurtman RJ (December 1971). "Brain serotonin content: increase following ingestion of carbohydrate diet". Science. 174 (4013): 1023–5. doi:10.1126/science.174.4013.1023. PMID 5120086.
- 1 2 Lyons PM, Truswell AS (March 1988). "Serotonin precursor influenced by type of carbohydrate meal in healthy adults" (PDF). The American Journal of Clinical Nutrition. 47 (3): 433–9. PMID 3279747.
- 1 2 3 Wurtman RJ, Wurtman JJ, Regan MM, McDermott JM, Tsay RH, Breu JJ (January 2003). "Effects of normal meals rich in carbohydrates or proteins on plasma tryptophan and tyrosine ratios". The American Journal of Clinical Nutrition. 77 (1): 128–32. PMID 12499331.
- 1 2 Afaghi A, O'Connor H, Chow CM (February 2007). "High-glycemic-index carbohydrate meals shorten sleep onset". The American Journal of Clinical Nutrition. 85 (2): 426–30. PMID 17284739.
- ↑ Pardridge WM, Oldendorf WH (August 1975). "Kinetic analysis of blood-brain barrier transport of amino acids". Biochimica et Biophysica Acta. 401 (1): 128–36. doi:10.1016/0005-2736(75)90347-8. PMID 1148286.
- ↑ Maher TJ, Glaeser BS, Wurtman RJ (May 1984). "Diurnal variations in plasma concentrations of basic and neutral amino acids and in red cell concentrations of aspartate and glutamate: effects of dietary protein intake". The American Journal of Clinical Nutrition. 39 (5): 722–9. PMID 6538743.
- 1 2 3 Shaw K, Turner J, Del Mar C (2002). Shaw KA, ed. "Tryptophan and 5-hydroxytryptophan for depression". The Cochrane Database of Systematic Reviews (1): CD003198. doi:10.1002/14651858.CD003198. PMID 11869656.
- 1 2 Ravindran AV, da Silva TL (September 2013). "Complementary and alternative therapies as add-on to pharmacotherapy for mood and anxiety disorders: a systematic review". Journal of Affective Disorders. 150 (3): 707–19. doi:10.1016/j.jad.2013.05.042. PMID 23769610.
- ↑ Soh NL, Walter GT (2011). "Tryptophan and depression: can diet alone be the answer?". Acta Neuropsychiatrica VL. 23 (1): 1601–5215;. doi:10.1111/j.1601-5215.2010.00508.x.
- ↑ Fernstrom JD (December 2012). "Effects and side effects associated with the non-nutritional use of tryptophan by humans". The Journal of Nutrition. 142 (12): 2236S–2244S. doi:10.3945/jn.111.157065. PMID 23077193.
- 1 2 Wurtman RJ, Hefti F, Melamed E (December 1980). "Precursor control of neurotransmitter synthesis". Pharmacological Reviews. 32 (4): 315–35. PMID 6115400.
- 1 2 Henderson HE, Devlin R, Peterson J, Brunzell JD, Hayden MR (December 1990). "Frameshift mutation in exon 3 of the lipoprotein lipase gene causes a premature stop codon and lipoprotein lipase deficiency". Molecular Biology & Medicine. 7 (6): 511–7. PMID 2077351.
- ↑ Kimura T, Bier DM, Taylor CL (December 2012). "Summary of workshop discussions on establishing upper limits for amino acids with specific attention to available data for the essential amino acids leucine and tryptophan". The Journal of Nutrition. 142 (12): 2245S–2248S. doi:10.3945/jn.112.160846. PMID 23077196.
- 1 2 Howland RH (June 2012). "Dietary supplement drug therapies for depression". Journal of Psychosocial Nursing and Mental Health Services. 50 (6): 13–6. doi:10.3928/02793695-20120508-06. PMID 22589230.
- ↑ Jackson RW (1930). "A synthesis of tryptophol" (PDF). Journal of Biological Chemistry. 88 (3): 659–662.
- ↑ Young SN (September 2013). "Acute tryptophan depletion in humans: a review of theoretical, practical and ethical aspects". Journal of Psychiatry & Neuroscience. 38 (5): 294–305. doi:10.1503/jpn.120209. PMC 3756112
 . PMID 23428157.
. PMID 23428157. - ↑ Young SN (2013). "The effect of raising and lowering tryptophan levels on human mood and social behaviour". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 368 (1615): 20110375. doi:10.1098/rstb.2011.0375. PMC 3638380
 . PMID 23440461.
. PMID 23440461. - 1 2 3 4 "Information Paper on L-tryptophan and 5-hydroxy-L-tryptophan". FU. S. Food and Drug Administration, Center for Food Safety and Applied Nutrition, Office of Nutritional Products, Labeling, and Dietary Supplements. 2001-02-01. Archived from the original on 2005-02-25. Retrieved 2012-02-08.
- ↑ Slutsker L, Hoesly FC, Miller L, Williams LP, Watson JC, Fleming DW (July 1990). "Eosinophilia-myalgia syndrome associated with exposure to tryptophan from a single manufacturer". Jama. 264 (2): 213–7. doi:10.1001/jama.264.2.213. PMID 2355442.
- ↑ Back EE, Henning KJ, Kallenbach LR, Brix KA, Gunn RA, Melius JM (April 1993). "Risk factors for developing eosinophilia myalgia syndrome among L-tryptophan users in New York". The Journal of Rheumatology. 20 (4): 666–72. PMID 8496862.
- ↑ Kilbourne EM, Philen RM, Kamb ML, Falk H (October 1996). "Tryptophan produced by Showa Denko and epidemic eosinophilia-myalgia syndrome". The Journal of Rheumatology. Supplement. 46: 81–8; discussion 89–91. PMID 8895184.
- ↑ Mayeno AN, Lin F, Foote CS, Loegering DA, Ames MM, Hedberg CW, Gleich GJ (December 1990). "Characterization of "peak E," a novel amino acid associated with eosinophilia-myalgia syndrome". Science. 250 (4988): 1707–8. doi:10.1126/science.2270484. PMID 2270484.
- ↑ Ito J, Hosaki Y, Torigoe Y, Sakimoto K (January 1992). "Identification of substances formed by decomposition of peak E substance in tryptophan". Food and Chemical Toxicology. 30 (1): 71–81. doi:10.1016/0278-6915(92)90139-C. PMID 1544609.
- ↑ Smith MJ, Garrett RH (November 2005). "A heretofore undisclosed crux of eosinophilia-myalgia syndrome: compromised histamine degradation". Inflammation Research. 54 (11): 435–50. doi:10.1007/s00011-005-1380-7. PMID 16307217.
- ↑ Mayeno AN, Gleich GJ (September 1994). "Eosinophilia-myalgia syndrome and tryptophan production: a cautionary tale". Trends in Biotechnology. 12 (9): 346–52. doi:10.1016/0167-7799(94)90035-3. PMID 7765187.
- ↑ Raphals P (November 1990). "Does medical mystery threaten biotech?". Science. 250 (4981): 619. doi:10.1126/science.2237411. PMID 2237411.
Further reading
- Wood RM, Rilling JK, Sanfey AG, Bhagwagar Z, Rogers RD (May 2006). "Effects of tryptophan depletion on the performance of an iterated Prisoner's Dilemma game in healthy adults". Neuropsychopharmacology. 31 (5): 1075–84. doi:10.1038/sj.npp.1300932. PMID 16407905.
- Sturtz R (2009). "what is the difference between L-Tryptophan and 5-HTP?". The Lidtke letter: 1.
External links
- "KEGG PATHWAY: Tryptophan metabolism - Homo sapiens". KEGG: Kyoto Encyclopedia of Genes and Genomes. 2006-08-23. Retrieved 2008-04-20.
- G.P. Moss. "Tryptophan Catabolism (early stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Retrieved 2008-04-20.
- G.P. Moss. "Tryptophan Catabolism (later stages)". Nomenclature Committee of the International Union of Biochemistry and Molecular Biology (NC-IUBMB). Retrieved 2008-04-20.
- B Mikkelson; DP Mikkelson (2007-11-22). "Turkey Causes Sleepiness". Urban Legends Reference Pages. Snopes.com. Retrieved 2008-04-20.
.svg.png)