Boronic acid
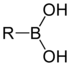
A boronic acid is an alkyl or aryl substituted boric acid containing a carbon–boron bond belonging to the larger class of organoboranes. Boronic acids act as Lewis acids. Their unique feature is that they are capable of forming reversible covalent complexes with sugars, amino acids, hydroxamic acids, etc. (molecules with vicinal, (1,2) or occasionally (1,3) substituted Lewis base donors (alcohol, amine, carboxylate)). The pKa of a boronic acid is ~9, but they can form tetrahedral boronate complexes with pKa ~7. They are occasionally used in the area of molecular recognition to bind to saccharides for fluorescent detection or selective transport of saccharides across membranes.
Boronic acids are used extensively in organic chemistry as chemical building blocks and intermediates predominantly in the Suzuki coupling. A key concept in its chemistry is transmetallation of its organic residue to a transition metal.
The compound bortezomib with a boronic acid group is a drug used in chemotherapy. The boron atom in this molecule is a key substructure because through it certain proteasomes are blocked that would otherwise degrade proteins.
Boronic acids
Many air-stable boronic acids are commercially available. They are characterised by high melting points. Since boronic acids easily lose water to form the cyclic trimeric anhydride, commercial material often contains substantial quantities of this anhydride. This does not affect reactivity.
| Boronic acid | R | Structure | Molar mass | CAS number | Melting point °C |
|---|---|---|---|---|---|
| Phenylboronic acid | Phenyl | 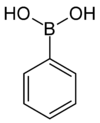 | 121.93 | 98-80-6 | 216–219 |
| 2-Thienylboronic acid | Thiophene | 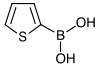 | 127.96 | 6165-68-0 | 138–140 |
| Methylboronic acid | Methyl | 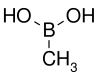 | 59.86 | 13061-96-6 | 91–94 |
| cis-Propenylboronic acid | propene | 85.90 | 7547-96-8 | 65–70 | |
| trans-Propenylboronic acid | propene |  | 85.90 | 7547-97-9 | 123–127 |
Synthesis
Boronic acids can be obtained via several methods. The most common way is reaction of organometallic compounds based on lithium or magnesium (Grignards) with borate esters.[1][2][3][4] For example, phenylboronic acid is produced from phenylmagnesium bromide and trimethyl borate followed by hydrolysis[5]
- PhMgBr + B(OMe)3 → PhB(OMe)2 + MeOMgBr
- PhB(OMe)2 + H2O → PhB(OH)2 + MeOH
Another method is reaction of an arylsilane (RSiR3) with boron tribromide (BBr3) in a transmetallation to RBBr2 followed by acidic hydrolysis.
A third method is by palladium catalysed reaction of aryl halides and triflates with diboronyl esters in a coupling reaction. An alternative to esters in this method is the use of diboronic acid or tetrahydroxydiboron ([B(OH2)]2).[6][7]
Boronic esters (also named boronate esters)
Boronic esters are esters formed between a boronic acid and an alcohol.
| Compound | General formula | General structure |
|---|---|---|
| Boronic acid | RB(OH)2 |  |
| Boronic ester | RB(OR)2 |  |
The compounds can be obtained from borate esters[8] by condensation with alcohols and diols. Phenylboronic acid can be selfcondensed to the cyclic trimer called triphenyl anhydride or triphenylboroxin.[9]
| Boronic ester | Diol | Structural formula | Molar mass | CAS number | Boiling point (°C) |
|---|---|---|---|---|---|
| Allylboronic acid pinacol ester | pinacol | 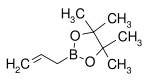 | 168.04 | 72824-04-5 | 50–53 (5 mmHg) |
| Phenyl boronic acid trimethylene glycol ester | trimethylene glycol |  | 161.99 | 4406-77-3 | 106 (2 mm Hg) |
| Diisopropoxymethylborane | isopropanol |  | 144.02 | 86595-27-9 | 105 -107 |
Compounds with 5-membered cyclic structures containing the C–O–B–O–C linkage are called dioxaborolanes and those with 6-membered rings dioxaborinanes.
Boronic acids in organic chemistry
Suzuki coupling reaction
Boronic acids are used in organic chemistry in the Suzuki reaction. In this reaction the boron atom exchanges its aryl group with an alkoxy group from palladium.
-
(1)
Chan–Lam coupling
In the Chan–Lam coupling the alkyl, alkenyl or aryl boronic acid reacts with a N–H or O–H containing compound with Cu(II) such as copper(II) acetate and oxygen and a base such as pyridine[10][11] forming a new carbon–nitrogen bond or carbon–oxygen bond for example in this reaction of 2-pyridone with trans-1-hexenylboronic acid:
The reaction mechanism sequence is deprotonation of the amine, coordination of the amine to the copper(II), transmetallation (transferring the alkyl boron group to copper and the copper acetate group to boron), oxidation of Cu(II) to Cu(III) by oxygen and finally reductive elimination of Cu(III) to Cu(I) with formation of the product. Direct reductive elimination of Cu(II) to Cu(0) also takes place but is very slow. In catalytic systems oxygen also regenerates the Cu(II) catalyst.
Liebeskind–Srogl coupling
In the Liebeskind–Srogl coupling a thiol ester is coupled with a boronic acid to produce a ketone.
Conjugate addition
The boronic acid organic residue is a nucleophile in conjugate addition also in conjunction with a metal. In one study the pinacol ester of allylboronic acid is reacted with dibenzylidene acetone in such a conjugate addition:[12]
- The catalyst system in this reaction is tris(dibenzylideneacetone)dipalladium(0) / tricyclohexylphosphine.
Another conjugate addition is that of gramine with phenylboronic acid catalyzed by cyclooctadiene rhodium chloride dimer:[13]
Oxidation
Boronic esters are oxidized to the corresponding alcohols with base and hydrogen peroxide (for an example see: carbenoid)
Homologation
- In boronic ester homologization an alkyl group shifts from boron in a boronate to carbon:[14]
 Boronic ester homologization
Boronic ester homologization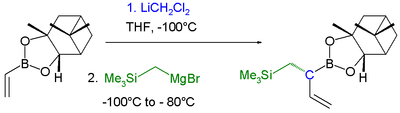 Homologization application
Homologization application
In this reaction dichloromethyllithium converts the boronic ester into a boronate. A Lewis acid then induces a rearrangement of the alkyl group with displacement of the chlorine group. Finally an organometallic reagent such as a Grignard reagent displaces the second chlorine atom effectively leading to insertion of an RCH2 group into the C-B bond. Another reaction featuring a boronate alkyl migration is the Petasis reaction.
Electrophilic allyl shifts
Allyl boronic esters engage in electrophilic allyl shifts very much like silicon pendant in the Sakurai reaction. In one study a diallylation reagent combines both[15][note 1]:
Hydrolysis
Hydrolysis of boronic esters back to the boronic acid and the alcohol can be accomplished in certain systems with thionyl chloride and pyridine.[16] Aryl boronic acids or esters may be hydrolyzed to the corresponding phenols by reaction with hydroxylamine at room temperature.[17]
C–H coupling reactions
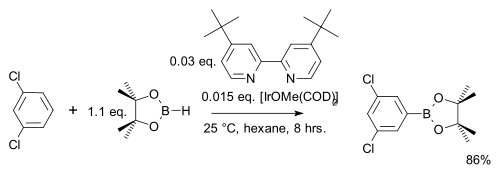
The diboron compound bis(pinacolato)diboron[18] reacts with aromatic heterocycles[19] or simple arenes[20] to an arylboronate ester with iridium catalyst [IrCl(COD)]2 (a modification of Crabtree's catalyst) and base 4,4′-di-tert-butyl-2,2′-bipyridine in a C-H coupling reaction for example with benzene:
In one modification the arene reacts 1 on 1 (instead of a large excess) with cheaper pinacolborane[21]
Unlike in ordinary electrophilic aromatic substitution (EAS) where electronic effects dominate, the regioselectivity in this reaction type is solely determined by the steric bulk of the iridium complex. This is exploited in a meta-bromination of m-xylene which by standard AES would give the ortho product[22][note 2]:
Protonolysis
Protodeboronation is a chemical reaction involving the protonolysis of a boronic acid (or other organoborane compound) in which a carbon-boron bond is broken and replaced with a carbon-hydrogen bond. Protodeboronation is a well-known undesired side reaction, and frequently associated with metal-catalysed coupling reactions that utilise boronic acids (see Suzuki reaction). For a given boronic acid, the propensity to undergo protodeboronation is highly variable and dependent on various factors, such as the reaction conditions employed and the organic substituent of the boronic acid:

Boronic acids in supramolecular chemistry
Saccharide recognition

The covalent pair-wise interaction between boronic acids and 1,2- or 1,3-diols in aqueous systems is rapid and reversible. As such the equilibrium established between boronic acids and the hydroxyl groups present on saccharides has been successfully employed to develop a range of sensors for saccharides.[23] One of the key advantages with this dynamic covalent strategy[24] lies in the ability of boronic acids to overcome the challenge of binding neutral species in aqueous media. If arranged correctly, the introduction of a tertiary amine within these supramolecular systems will permit binding to occur at physiological pH and allow signalling mechanisms such as photoinduced electron transfer mediated fluorescence emission to report the binding event.
Potential applications for this research include systems to monitor diabetic blood glucose levels. As the sensors employ an optical response, monitoring could be achieved using minimally invasive methods, one such example is the investigation of a contact lens doped with boronic acid based sensors to monitor glucose levels within ocular fluid.[25]
Borinic acids and esters
Borinic acids and borinate esters have the general structure R2BOR.
| compound | general formula | general structure |
|---|---|---|
| borinic acid | R2BOH |  |
| borinate ester | R2BOR |  |
Borate salts
Borate salts are ate complexes and have the general structure R4B−M+ for example potassium tetraphenylborate (IUPAC name: potassium tetraphenylboranuide).
Notes
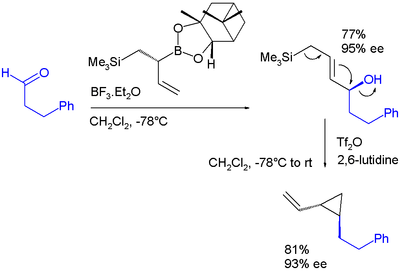
- ↑ In this sequence the boronic ester allyl shift is catalyzed by boron trifluoride. In the second step the hydroxyl group is activated as a leaving group by conversion to a triflate by triflic anhydride aided by 2,6-lutidine. The final product is a vinyl cyclopropane. Note: ee stands for enantiomeric excess
- ↑ In situ second step reaction of boronate ester with copper(II) bromide
See also
- Boron bonded to three oxygen atoms: boric acid and borates
- Suzuki reaction
- Supramolecular chemistry
- Dynamic covalent chemistry
- Blood glucose monitoring
References
- ↑ Boronic Acids. Edited by Dennis G. Hall 2005 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim ISBN 3-527-30991-8
- ↑ Example: Jesper Langgaard Kristensen, Morten Lysén, Per Vedsø, and Mikael Begtrup Günter Seidel and Alois Fürstner Published in Org. Synth. 2005, 81, 134 Org. Synth. 2009, Coll. Vol. 11, 1015 link Archived 22 March 2012 at the Wayback Machine.
- ↑ Example: Quinoline, 3-(3-pyridinyl)- Wenjie Li, Dorian P. Nelson, Mark S. Jensen, R. Scott Hoerrner, Dongwei Cai, and Robert D. Larsen, Scott E. Denmark, Geoff T. Halvorsen, and Jeffrey M. Kallemeyn Published in Org. Synth. 2005, 81, 89 Org. Synth. 2009, Coll. Vol. 11, 393 Link Archived 22 March 2012 at the Wayback Machine.
- ↑ "Cyclopropanemethanol, 2-phenyl-, (1S'-trans)-" André B. Charette and Hélène Lebel Kevin Minbiole, Patrick Verhoest, and Amos B. Smith, III Published in Org. Synth. 1999, 76, 86 Org. Synth. 2004, Coll. Vol. 10, 613 Link Archived 22 March 2012 at the Wayback Machine.
- ↑ Wahsburn, R. M.; Levens, E.; Albright, C. F.; Billig, F. A. (1963). "Benzeneboronic anhydride". Org. Synth.; Coll. Vol., 4, p. 68
- ↑ Pilarski, L. T. and Szabó, K. J. (2011), Palladium-Catalyzed Direct Synthesis of Organoboronic Acids. Angewandte Chemie International Edition, 50: 8230–8232. doi:10.1002/anie.201102384
- ↑ Palladium-Catalyzed, Direct Boronic Acid Synthesis from Aryl Chlorides: A Simplified Route to Diverse Boronate Ester Derivatives Gary A. Molander, Sarah L. J. Trice, Spencer D. Dreher Journal of the American Chemical Society 2010 132 (50), 17701-17703 doi:10.1021/ja1089759
- ↑ R. L. Kidwell, M. Murphy, and S. D. Darling (1973). "Phenols: 6-Methoxy-2-Naphthol". Org. Synth.; Coll. Vol., 5, p. 918
- ↑ Robert M. Washburn, Ernest Levens, Charles F. Albright, and Franklin A. Billig (1963). "Benzeneboronic Anhydride". Org. Synth.; Coll. Vol., 4, p. 68
- ↑ Copper promoted C-N and C-O bond cross-coupling with phenyl and pyridylboronatesTetrahedron Letters, Volume 44, Issue 19, 5 May 2003, Pages 3863–3865 Dominic M. T. Chan, Kevin L. Monaco, Renhua Li, Damien Bonne, Charles G. Clark and Patrick Y. S. Lam doi:10.1016/S0040-4039(03)00739-1
- ↑ Copper-promoted/catalyzed C-N and C-O bond cross-coupling with vinylboronic acid and its utilities Tetrahedron Letters, Volume 44, Issue 26, 23 June 2003, Pages 4927–4931 Patrick Y. S. Lam, Guillaume Vincent, Damien Bonne and Charles G. Clark doi:10.1016/S0040-4039(03)01037-2
- ↑ Catalytic Conjugate Addition of Allyl Groups to Styryl-Activated Enones Joshua D. Sieber, Shubin Liu, and James P. Morken J. Am. Chem. Soc.; 2007; 129(8) pp 2214–2215; (Communication) doi:10.1021/ja067878w
- ↑ Benzylic Substitution of Gramines with Boronic Acids and Rhodium or Iridium Catalysts Gabriela de la Herrán, Amaya Segura, and Aurelio G. Csák Org. Lett.; 2007; 9(6) pp 961 – 964; (Letter) doi:10.1021/ol063042m
- ↑ 99% Chirally selective synthesis via pinanediol boronic esters: insect pheromones, diols, and an amino alcohol Donald S. Matteson, Kizhakethil Mathew Sadhu, and Mark L. Peterson J. Am. Chem. Soc.; 1986; 108(4); pp 810 – 819; doi:10.1021/ja00264a039
- ↑ Simple, Stable, and Versatile Double-Allylation Reagents for the Stereoselective Preparation of Skeletally Diverse Compounds Feng Peng and Dennis G. Hall J. Am. Chem. Soc.; 2007; 129(11) pp 3070 – 3071; (Communication) doi:10.1021/ja068985t
- ↑ New asymmetric syntheses with boronic esters and fluoroboranes Donald S. Matteson Pure Appl. Chem., Vol. 75, No. 9, pp. 1249–1253, 2003 Link.
- ↑ A mild conversion of arylboronic acids and their pinacolyl boronate esters into phenols using hydroxylamine Ebrahim Kianmehr , Tetrahedron Letters ,vol. 48 , Issue 15 , 9 April 2007
- ↑ Tatsuo Ishiyama, Miki Murata, Taka-aki Ahiko, and Norio Miyaura (2004). "Bis(pinacolato)diboron". Org. Synth.; Coll. Vol., 10, p. 115
- ↑ Iridium-catalyzed C–H coupling reaction of heteroaromatic compounds with bis(pinacolato)diboron: regioselective synthesis of heteroarylboronates Tetrahedron Letters, Volume 43, Issue 32, 5 August 2002, Pages 5649–5651 Jun Takagi, Kazuaki Sato, John F. Hartwig, Tatsuo Ishiyama and Norio Miyaura doi:10.1016/S0040-4039(02)01135-8
- ↑ Mild Iridium-Catalyzed Borylation of Arenes. High Turnover Numbers, Room Temperature Reactions, and Isolation of a Potential Intermediate Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N. R.; Hartwig, J. F. J. Am. Chem. Soc. (Communication); 2002; 124(3); 390–391. doi:10.1021/ja0173019
- ↑ Room temperature borylation of arenes and heteroarenes using stoichiometric amounts of pinacolborane catalyzed by iridium complexes in an inert solvent Tatsuo Ishiyama, Yusuke Nobuta, John F. Hartwig and Norio Miyaura Chem. Commun. 2003, 2924–2925, doi:10.1039/b311103b
- ↑ Meta Halogenation of 1,3-Disubstituted Arenes via Iridium-Catalyzed Arene Borylation Jaclyn M. Murphy, Xuebin Liao, and John F. Hartwig J. AM. CHEM. SOC. 2007, 129, 15434-15435 doi:10.1021/ja076498n
- ↑ Boronic Acids in Saccharide Recognition, Tony D. James, Marcus D. Phillips and Seiji Shinkai, Royal Society of Chemistry (2006) ISBN 978-0-85404-537-2 doi:10.1039/9781847557612
- ↑ Stuart J. Rowan, Stuart J. Cantrill, Graham R. L. Cousins, Jeremy K. M. Sanders, J. Fraser Stoddart (2002). "Dynamic Covalent Chemistry". Angewandte Chemie International Edition 41 (6): 898–952 doi:10.1002/1521-3773(20020315)41:6<898::AID-ANIE898>3.0.CO;2-E PMID 12491278
- ↑ U.S. Patent No. 6850786, filed 3 September 2003.
External links
| Wikimedia Commons has media related to Supramolecular chemistry. |