Chirality (chemistry)
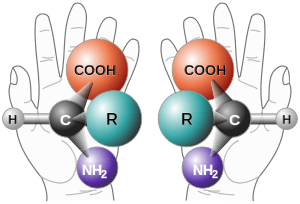
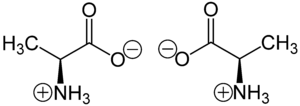
Chirality /kaɪˈrælɪti/ is a geometric property of some molecules and ions. A chiral molecule/ion is non-superposable on its mirror image. The presence of an asymmetric carbon center is one of several structural features that induce chirality in organic and inorganic molecules.[1][2][3][4] The term chirality is derived from the Greek word for hand, χειρ (kheir).
The mirror images of a chiral molecule/ion are called enantiomers or optical isomers. Individual enantiomers are often designated as either "right-" or "left-handed". Chirality is an essential consideration when discussing the stereochemistry in inorganic chemistry and organic chemistry. The concept is of great practical importance because most biomolecules and pharmaceuticals are chiral.
Chiral molecules and ions are described by various ways of designating their absolute configuration, which codify either the entity's geometry or its ability to rotate plane-polarized light, a common technique in studying chirality.
Definition
Chirality is based on molecular symmetry elements. Specifically, a chiral compound can contain no improper axis of rotation (Sn), which includes planes of symmetry and inversion center. Chiral molecules are always dissymmetric (lacking Sn) but not always asymmetric (lacking all symmetry elements except the trivial identity). Asymmetric molecules are always chiral.[5]
| Rotational axis (Cn) | Improper rotational elements (Sn) | ||
|---|---|---|---|
| Chiral no Sn | Achiral mirror plane S1 = σ | Achiral inversion centre S2 = i | |
| C1 | 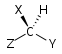 | 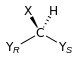 |  |
| C2 |  | 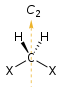 |  |
Stereogenic centers
In general, chiral molecules have point chirality at a single stereogenic atom, which has four different substituents. The two enantiomers of such compounds are said to have different absolute configurations at this center. This center is thus stereogenic (i.e., a grouping within a molecular entity that may be considered a focus of stereoisomerism). The stereogenic atom is usually carbon, as in many biological molecules. However chirality can exist in any atom, including metals (as in many chiral coordination compounds), phosphorus, or sulfur. Chiral nitrogen is equally possible, although the effects of nitrogen inversion can make many of these compounds impossible to isolate.
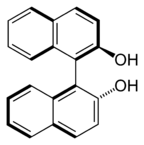
While the presence of a stereogenic atom describes the great majority of cases, many variations and exceptions exist. For instance it is not necessary for the chiral substance to have a stereogenic atom. Examples include 1-bromo-1-chloro-1-fluoroadamantane, methylethylphenyltetrahedrane, certain calixarenes and fullerenes, which have inherent chirality. The C2-symmetric species 1,1'-bi-2-naphthol (BINOL), 1,3-dichloro-allene have axial chirality. (E)-cyclooctene and many ferrocenes have planar chirality.
When the optical rotation for an enantiomer is too low for practical measurement, the species is said to exhibit cryptochirality.
Even isotopic differences must be considered when examining chirality. Illustrative is the derivative of benzyl alcohol PhCHDOH is chiral. The S enantiomer has [α]D = +0.715°.[6]
In biochemistry
Many biologically active molecules are chiral, including the naturally occurring amino acids (the building blocks of proteins) and sugars. In biological systems, most of these compounds are of the same chirality: most amino acids are levorotatory (L) and sugars are dextrorotatory (D). Typical naturally occurring proteins, made of L amino acids, are known as left-handed proteins, whereas D amino acids produce right-handed proteins . D-amino acids are very rare in nature and have only been found in small peptides attached to bacteria cell walls.
The origin of this homochirality in biology is the subject of much debate.[7] Most scientists believe that Earth life's "choice" of chirality was purely random, and that if carbon-based life forms exist elsewhere in the universe, their chemistry could theoretically have opposite chirality. However, there is some suggestion that early amino acids could have formed in comet dust. In this case, circularly polarised radiation (which makes up 17% of stellar radiation) could have caused the selective destruction of one chirality of amino acids, leading to a selection bias which ultimately resulted in all life on Earth being homochiral.[8][9]
Enzymes, which are chiral, often distinguish between the two enantiomers of a chiral substrate. One could imagine an enzyme as having a glove-like cavity that binds a substrate. If this glove is right-handed, then one enantiomer will fit inside and be bound, whereas the other enantiomer will have a poor fit and is unlikely to bind.
L-forms of amino acids tend to be tasteless, whereas D-forms tend to taste sweet.[7] Spearmint leaves contain the L-enantiomer of the chemical carvone or R-(–)-carvone and caraway seeds contain the D-enantiomer or S-(+)-carvone.[10] These smell different to most people because our olfactory receptors are chiral.
Chirality is important in context of ordered phases as well, for example the addition of a small amount of an optically active molecule to a nematic phase (a phase that has long range orientational order of molecules) transforms that phase to a chiral nematic phase (or cholesteric phase). Chirality in context of such phases in polymeric fluids has also been studied in this context.[11]
In inorganic chemistry
-cation-3D-balls.png)
Chirality is a symmetry property, not a characteristic of any part of the periodic table. Thus many inorganic materials, molecules, and ions are chiral. Quartz is an example from the mineral kingdom. Such noncentric materials are of interest for applications in nonlinear optics.
In the areas of coordination chemistry and organometallic chemistry, chirality is pervasive and of practical importance. A famous example is tris(bipyridine)ruthenium(II) complex in which the three bipyridine ligands adopt a chiral propeller-like arrangement.[12] The two enantiomers of complexes such as [Ru(2,2′-bipyridine)3]2+ may be designated as Λ (capital lambda, the Greek version of "L") for a left-handed twist of the propeller described by the ligands, and Δ (capital delta, Greek "D") for a right-handed twist (pictured).
Chiral ligands confer chirality to a metal complex, as illustrated by metal-amino acid complexes. If the metal exhibits catalytic properties, its combination with a chiral ligand is the basis of asymmetric catalysis.[13]
Methods and practices
The term optical activity is derived from the interaction of chiral materials with polarized light. In a solution, the (−)-form, or levorotatory form, of an optical isomer rotates the plane of a beam of linearly polarized light counterclockwise. The (+)-form, or dextrorotatory form, of an optical isomer does the opposite. The rotation of light is measured using a polarimeter and is expressed as the optical rotation.
Miscellaneous nomenclature
- Any non-racemic chiral substance is called scalemic. Scalemic materials can be enantiopure or enantioenriched.[14]
- A chiral substance is enantiopure when only one of two possible enantiomers is present so that all molecules within a sample have the same chirality sense. Use of homochiral as a synonym is strongly discouraged.[15]
- A chiral substance is enantioenriched or heterochiral when its enantiomeric ratio is greater than 50:50 but less than 100:0.[16]
- Enantiomeric excess or ee is the difference between how much of one enantiomer is present compared to the other. For example, a sample with 40% ee of R contains 70% R and 30% S (70% − 30% = 40%).[17]
History
The rotation of plane polarized light by chiral substances was first observed by Jean-Baptiste Biot in 1815,[18] and gained considerable importance in the sugar industry, analytical chemistry, and pharmaceuticals. Louis Pasteur deduced in 1848 that this phenomenon has a molecular basis.[19][20] The term chirality itself was coined by Lord Kelvin in 1894.[21] Different enantiomers or diastereomers of a compound were formerly called optical isomers due to their different optical properties.[22] At one time, chirality was thought to be associated with organic chemistry, but this misconception was overthrown by the resolution of a purely inorganic compound, hexol, by Alfred Werner.
See also
- Chemical chirality in popular fiction
- Chirality (mathematics)
- Chirality (physics)
- Enantioselective synthesis
- Pfeiffer effect
- Stereochemistry for overview of stereochemistry in general
- Stereoisomerism
- Supramolecular chirality
References
- ↑ Organic Chemistry (4th Edition) Paula Y. Bruice. Pearson Educational Books. ISBN 9780131407480
- ↑ Organic Chemistry (3rd Edition) Marye Anne Fox, James K. Whitesell Jones & Bartlett Publishers (2004) ISBN 0763721972
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Chirality".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Superposability".
- ↑ Cotton, F. A., "Chemical Applications of Group Theory," John Wiley & Sons: New York, 1990.
- ↑ ^ Streitwieser, A., Jr.; Wolfe, J. R., Jr.; Schaeffer, W. D. (1959). "Stereochemistry of the Primary Carbon. X. Stereochemical Configurations of Some Optically Active Deuterium Compounds". Tetrahedron. 6 (4): 338–344. doi:10.1016/0040-4020(59)80014-4.
- 1 2 Meierhenrich, Uwe J. (2008). Amino acids and the Asymmetry of Life. Berlin, GER: Springer. ISBN 3540768858.
- ↑ McKee, Maggie (2005-08-24). "Space radiation may select amino acids for life". New Scientist. Retrieved 2016-02-05.
- ↑ Uwe J. Meierhenrich, Laurent Nahon, Christian Alcaraz, Jan Hendrik Bredehöft, Søren V. Hoffmann, Bernard Barbier and André Brack "Asymmetric Vacuum UV photolysis of the Amino Acid Leucine in the Solid State" Angew. Chem. Int. Ed. 2005, volume 44, 5630–5634. doi:10.1002/anie.200501311
- ↑ Theodore J. Leitereg; Dante G. Guadagni; Jean Harris; Thomas R. Mon; Roy Teranishi (1971). "Chemical and sensory data supporting the difference between the odors of the enantiomeric carvones". J. Agric. Food Chem. 19 (4): 785–787. doi:10.1021/jf60176a035.
- ↑ Srinivasarao, M. (1999). "Chirality and Polymers". Current Opinion in Colloid and Interface Science. 4 (5): 369–376.
- ↑ von Zelewsky, A. (1995). Stereochemistry of Coordination Compounds. Chichester: John Wiley.. ISBN 047195599X.
- ↑ Hartwig, J. F. Organotransition Metal Chemistry, from Bonding to Catalysis; University Science Books: New York, 2010. ISBN 189138953X
- ↑ Eliel, E.L. (1997). "Infelicitous Stereochemical Nomenclatures". Chirality. 9: 428–430. Retrieved 5 February 2016.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "asymmetric synthesis".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomerically enriched (enantioenriched)".
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "enantiomer excess (enantiomeric excess)".
- ↑ Lakhtakia, A. (ed.) (1990). Selected Papers on Natural Optical Activity (SPIE Milestone Volume 15). SPIE.
- ↑ Pasteur, L. (1848). "Researches on the molecular asymmetry of natural organic products, English translation of French original, published by Alembic Club Reprints (Vol. 14, pp. 1–46) in 1905, facsimile reproduction by SPIE in a 1990 book".
- ↑ Eliel, Ernest Ludwig; Wilen, Samuel H. & Mander, Lewis N. (1994). "Chirality in Molecules Devoid of Chiral Centers (Chapter 14)". Stereochemistry of Organic Compounds (1st ed.). New York, NY, USA: Wiley & Sons. ISBN 0471016705. Retrieved 2 February 2016.
- ↑ Bentley, Ronald (1995). "From Optical Activity in Quartz to Chiral Drugs: Molecular Handedness in Biology and Medicine.". Perspect. Biol. Med. 38 (2): 188–229. doi:10.1353/pbm.1995.0069. PMID 7899056.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "Optical isomers".
Further reading
- Clayden, Jonathan ; Greeves, Nick & Warren, Stuart (2012). Organic Chemistry (2nd ed.). Oxford, UK: Oxford University Press. pp. 319f, 432, 604np, 653, 746int, 803ketals, 839, 846f. ISBN 0199270295. Retrieved 2 February 2016.
- Eliel, Ernest Ludwig; Wilen, Samuel H. & Mander, Lewis N. (1994). "Chirality in Molecules Devoid of Chiral Centers (Chapter 14)". Stereochemistry of Organic Compounds (1st ed.). New York, NY, USA: Wiley & Sons. doi:10.1002/(SICI)1520-636X(1997)9:5/6<428::AID-CHIR5>3.0.CO;2-1. ISBN 0471016705. Retrieved 2 February 2016.
- Eliel, E.L. (1997). "Infelicitous Stereochemical Nomenclatures". chirality. 9: 428–430. doi:10.1002/(SICI)1520-636X(1997)9:5/6<428::AID-CHIR5>3.0.CO;2-1. Retrieved 5 February 2016.
- Gal, Joseph (2013). "Molecular Chirality: Language, History, and Significance". Differentiation of Enantiomers I. chirality. Topics in Current Chemistry. 340. pp. 1–20. Retrieved 5 February 2016.
External links
| Wikimedia Commons has media related to Chirality. |
- 21st International Symposium on Chirality
- STEREOISOMERISM - OPTICAL ISOMERISM
- Symposium highlights-Session 5: New technologies for small molecule synthesis
- IUPAC nomenclature for amino acid configurations.
- Michigan State University's explanation of R/S nomenclature
- Chirality & Odour Perception at leffingwell.com
- Chirality & Bioactivity I.: Pharmacology
- Chirality and the Search for Extraterrestrial Life
- The Handedness of the Universe by Roger A Hegstrom and Dilip K Kondepudi http://quantummechanics.ucsd.edu/ph87/ScientificAmerican/Sciam/Hegstrom_The_Handedness_of_the_universe.pdf