Cryptochirality
Cryptochirality in stereochemistry is a special case of chirality where due to the electronic properties of the chiral molecule its specific rotation is non-measurable. The term was introduced by K. Mislow in 1977.
For example the alkane 5-ethyl-5-propylundecane found in certain species of Phaseolus vulgaris has two enantiomeric forms without any observable optical rotation:[1]
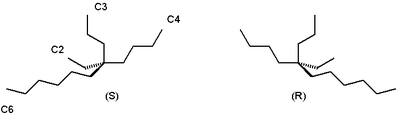
It is still possible to distinguish between the two enantiomers by using them control the asymmetric synthesis of another chemical whose stereochemical nature can be measured. For example, the Soai reaction of 2-(3,3-dimethylbut-1-ynyl)pyrimidine-5-carbaldehyde (1) with diisopropylzinc performed in the presence of 3 forms secondary alcohol 4 with a high enantiomeric excess based on the major enantiomer of 3 that was used. Even a slight enantiomeric excess of 3 is rapidly amplified due to the autocatalytic nature of this reaction:
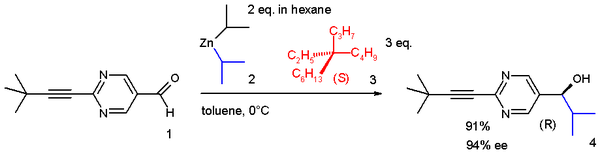
The chiral induction is believed occur as a result of interactions between the C–H bonds of the alkane and the pi electrons of the aldehyde.
Cryptochirality also occurs in polymeric systems growing from chiral initiators for example in dendrimers with lobes of different size attached to a central core.[2]
See also
- More on diisopropylzinc autocatalysis see: homochirality
References
- ↑ Chiral Discrimination of Cryptochiral Saturated Quaternary and Tertiary Hydrocarbons by Asymmetric Autocatalysis Kawasaki, T.; Tanaka, H.; Tsutsumi, T.; Kasahara, T.; Sato, I.; Soai, K. J. Am. Chem. Soc.; 2006; 128(18); 6032-6033. doi:10.1021/ja061429e
- ↑ Cryptochirality and dendrimers Struijk, MP Peerlings, HWI Meijer, EW Polymer Preprints 37(2), 497-498 (1996) Article