Calixarene
A calixarene is a macrocycle or cyclic oligomer based on a hydroxyalkylation product of a phenol and an aldehyde.[1][2]
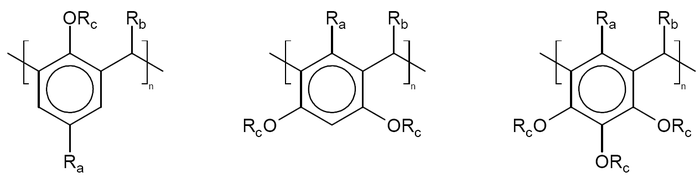
The word calixarene is derived from calix or chalice because this type of molecule resembles a vase and from the word arene that refers to the aromatic building block. Calixarenes have hydrophobic cavities that can hold smaller molecules or ions and belong to the class of cavitands known in host-guest chemistry. Calixarene nomenclature is straightforward and involves counting the number of repeating units in the ring and include it in the name. A calix[4]arene has 4 units in the ring and a calix[6]arene has 6. A substituent in the meso position Rb is added to the name with a prefix C- as in C-methylcalix[6]arene.
Synthesis
The aromatic components are derived from phenol, resorcinol, or pyrogallol. For phenol, the aldehyde most often used is simple formaldehyde, while larger aldehydes, like acetaldehyde, are usually required in condensation reactions with resorcinol and pyrogallol. The chemical reaction qualifies as electrophilic aromatic substitution, followed by an elimination of water, and then a second aromatic substitution. The reaction is catalyzed by acids or bases.
Calixarenes are difficult to produce because random polymerization occurs inside of complex mixtures of linear and cyclic oligomers with different numbers of repeating units. With finely tuned starting materials and reaction conditions, synthesis can also be surprisingly facile. In 2005, researchers produced pyrogallol[4]arene simply by mixing a solvent-free dispersion of isovaleraldehyde with pyrogallol, and a catalytic amount of p-toluenesulfonic acid, in a mortar and pestle.[3] Calixarenes are sparingly soluble as parent compounds and melt at high temperatures compared to other crystalline solids.[4]
Structure
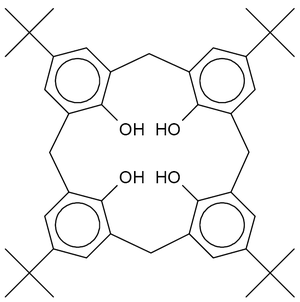
Calixarenes are characterised by a three-dimensional basket, cup or bucket shape. In calix[4]arenes the internal volume is around 10 cubic angstroms. Calixarenes are characterised by a wide upper rim and a narrow lower rim and a central annulus. With phenol as a starting material the 4 hydroxyl groups are intrannular on the lower rim. In a resorcin[4]arene 8 hydroxyl groups are placed extraannular on the upper ring. Calixarenes exist in different chemical conformations because rotation around the methylene bridge is not difficult. In calix[4]arene 4 up-down conformations exist: cone (point group C2v,C4v), partial cone Cs, 1,2 alternate C2h and 1,3 alternate D2d. The 4 hydroxyl groups interact by hydrogen bonding and stabilize the cone conformation. This conformation is in dynamic equilibrium with the other conformations. Conformations can be locked in place with proper substituents replacing the hydroxyl groups which increase the rotational barrier. Alternatively placing a bulky substituent on the upper rim also locks a conformation. The calixarene based on p-tert-butyl phenol is also a cone.[5] Calixarenes are structurally related to the pillararenes.
 |
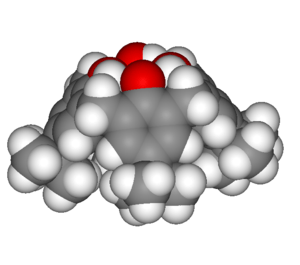 |
| Calix[4]arene with para-tert-butyl substituents | 3D representation of a cone conformation |
History
In 1872 Adolf von Baeyer mixed various aldehydes, including formaldehyde, with phenols in a strongly acidic solution. The resultant tars defied characterization; but represented the typical products of a phenol/formaldehyde polymerization. Leo Baekeland, Belgian by birth and a naturalized American citizen, discovered that these tars could be cured into a brittle substance which he marketed as “Bakelite”. This polymer was the first commercial synthetic plastic.
The success of Bakelite spurred scientific investigations into the chemistry of the phenol/formaldehyde reaction. One result was the discovery made in 1942 by the Austrian chemist Alois Zinke, that p-alkyl phenols and formaldehyde in a strongly basic solution yield mixtures containing cyclic tetramers. Concomitantly, the American chemists Joseph Niederl and H. J. Vogel obtained similar cyclic tetramers from the acid-catalyzed reaction of resorcinol and aldehydes such as benzaldehyde. A number of years later, the British chemist John Cornforth showed that the product from p-tert-butylphenol and formaldehyde is a mixture of the cyclic tetramer and another ambiguous cyclomer. His interest in these compounds was in the tuberculostatic properties of their oxyethylated derivatives.
In the early 1970s the American chemist C. David Gutsche recognized the calix shape of the cyclic tetramer and thought that it might furnish the structure for building an enzyme xenologue. He initiated a study that lasted for three decades. His attention to these compounds came from acquaintance with the Petrolite company’s commercial demulsifiers, made by oxyethylation of the still ambiguous products from p-alkylphenols and formaldehyde. He introduced the name “calixarene”: from “calix”, the Greek name for a chalice, and “arene” for the presence of aryl groups in the cyclic array. He also determined the structures for the cyclic tetramer, hexamer, and octamer, along with procedures for obtaining these materials in good to excellent yields. He then established procedures for attaching functional groups to both the upper and lower rims and mapped the conformational states of these flexible molecules. Additionally, he proved that the cyclic tetramer can be frozen into a cone conformation, by the addition of measurably large substituents to the lower rim of the calix shape.
Concomitant with Gutsche’s work was that of the German chemists Hermann Kämmerer and Volker Böhmer. They developed methods for the stepwise synthesis of calixarenes. The Italian chemists Giovanni Andreetti, Rocco Ungaro and Andrea Pochini were the first to resolve x-ray crystallographic images of calixarenes. In the mid 1980s, other groups of investigators joined the field of calixarene chemistry. It has become an important aspect of supramolecular chemistry and attracts the attention of hundreds of scientists around the world. The Niederl cyclic tetramers from resorcinol and aldehydes were studied in detail by the American chemist Donald J. Cram, who called the derived compounds “cavitands” and “carcerands”. An accurate and detailed history of the calixarenes along with extensive discussion of calixarene chemistry can be found in the 1989 publication (ref 1) as well as the second edition in 2008
Host guest interactions
Calixarenes are efficient sodium ionophores and are applied as such in chemical sensors. With the right chemistry these molecules exhibit great selectivity towards other cations. Calixarenes are used in commercial applications as sodium selective electrodes for the measurement of sodium levels in blood. Calixarenes also form complexes with cadmium, lead, lanthanides and actinides. Calix[5]arene and the C70 fullerene in p-xylene form a ball-and-socket supramolecular complex.[6] Calixarenes also form exo-calix ammonium salts with aliphatic amines such as piperidine.[7]
Molecular self-assembly
Molecular self-assembly of resorcinarenes and pyrogallolarenes led to larger supramolecular assemblies. Both in the crystalline state and in solution, they are known to form hexamers that are akin to certain Archimedean solids with an internal volume of around one cubic nanometer (nanocapsules). (Isobutylpyrogallol[4]arene)6 is held together by 48 intermolecular hydrogen bonds. The remaining 24 hydrogen bonds are intramolecular. The cavity is filled by a number of solvent molecules.[8]
Catalysis
Calixarenes in general, and more specifically calix[4]arenes have been extensively used as molecular platform to build up supramolecular catalysts. The design of this kind of catalysts consists in functionalizing the upper rim of the lower rim of calixarenes with ligands able to bind metal cations, notably Cu(II) or Zn(II), or other active functions. These compounds are active in the catalysis of hydrolytic reactions.[9][10]
Other Applications
Calixarenes are applied in enzyme mimetics, ion sensitive electrodes or sensors, selective membranes, non-linear optics[11] and in HPLC stationary phases. In addition, in nanotechnology calixarenes are used as negative resist for high-resolution electron beam lithography .
A tetrathia[4]arene is found to mimic aquaporin proteins.[12] This calixarene adopts a 1,3-alternate conformation (methoxy groups populate the lower ring) and water is not contained in the basket but grabbed by two opposing tert-butyl groups on the outer rim in a pincer. The nonporous and hydrophobic crystals are soaked in water for 8 hours in which time the calixarene:water ratio nevertheless acquires the value of one.
Calixarenes are able to accelerate reactions taking place inside the concavity by a combination of local concentration effect and polar stabilization of the transition state. An extended resorcin[4]arene cavitand is found to accelerate the reaction rate of a Menshutkin reaction between quinuclidine and butylbromide by a factor of 1600.[13]
In heterocalixarenes the phenolic units are replaced by heterocycles,[14] for instance by furans in calix[n]furanes and by pyridines in calix[n]pyridines. Calixarenes have been used as the macrocycle portion of a rotaxane and two calixarene molecules covalently joined together by the lower rims form carcerands.
Calix[4]arene was used as scaffold to assemble a construct bearing four Tn-antigen unit, at upper rim, and the immunoadjuvant P3CS, at lower rim. The construct showed a cluster effect in the production of Tn specific IgG antibodies in mice when compared to an analogous monovalent construct. This reveals perspectives for potential applications in cancer immunotherapy.[15][16]
Inherent chirality
Calix[4]arenes with XXYZ or WXYZ substitution patterns at the upper rim are inherently chiral and their enantiomers can be resolved by chiral column chromatography. Recently, inherently chiral calixarenes have been synthesised in good yields by asymmetric ortholithiation using a chiral oxazoline directing group. This removes the need for resolution techniques.
References
- ↑ Gutsche, C. David (1989). Calixarenes. Cambridge: Royal Society of Chemistry. ISBN 0-85186-385-X.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (1995) "Calixarenes".
- ↑ Antesberger J, Cave GW, Ferrarelli MC, Heaven MW, Raston CL, Atwood JL (2005). "Solvent-free, direct synthesis of supramolecular nano-capsules". Chemical communications (Cambridge, England). . (7): 892–894. doi:10.1039/b412251h. PMID 15700072.
- ↑ McMahon G; O’Malley S; Nolan K; Diamond D (2003). "Important Calixarene Derivatives – their Synthesis and Applications". Arkivoc. Part (vii): 23–31. ISSN 1551-7012. Retrieved 2011-10-10.
- ↑
- ↑ Atwood, Jerry L.; Barbour, Leonard J.; Heaven, Michael W.; Raston, Colin L. (2003-09-01). "Association and orientation of C70 on complexation with calix[5]arene". Chemical Communications (18): 2270–2271. doi:10.1039/B306411P. PMID 14518869. Retrieved 2011-10-10.
- ↑ Nachtigall FF, Lazzarotto M, Braz FN (2002). "Interaction of Calix[4]arene and Aliphatic Amines: A Combined NMR, Spectrophotometric and Conductimetric Investigation". Journal of the Brazilian Chemical Society. 13 (3): 295–299. doi:10.1590/S0103-50532002000300002.
- ↑ Atwood JL, Barbour LJ, Jerga A (2002). "Organization of the interior of molecular capsules by hydrogen bonding". Proceedings of the National Academy of Sciences. 99 (8): 4837–41. doi:10.1073/pnas.082659799. PMC 122679
 . PMID 11943875.
. PMID 11943875. - ↑ Reactivity of carbonyl and phosphoryl groups at calixarenes R. Cacciapaglia, S. Di Stefano, L. Mandolini and R. Salvio; Supramol. Chem. 2013, 25, 537-554 doi:10.1080/10610278.2013.824578
- ↑ Calixarenes and resorcinarenes as scaffolds for supramolecular metallo-enzyme mimicry J.-N. Rebilly, O. Reinaud; Supramol. Chem. 2014, 1-27 doi:10.1080/10610278.2013.877137
- ↑ Hennrich, Gunther; Murillo, M. Teresa; Prados, Pilar; Song, Kai; Asselberghs, Inge; Clays, Koen; Persoons, André; Benet-Buchholz, Jordi; de Mendoza, Javier (2005-07-07). "Tetraalkynyl calix[4]arenes with advanced NLO properties". Chemical Communications (21): 2747–2749. doi:10.1039/B502045J. PMID 15917941. Retrieved 2011-10-10.
- ↑ Thallapally PK, Lloyd GO, Atwood JL, Barbour LJ (2005-06-20). "Diffusion of water in a nonporous hydrophobic crystal". Angewandte Chemie International Edition in English. 44 (25): 3848–3851. doi:10.1002/anie.200500749. PMID 15892031.
- ↑ Purse, BW; Gissot, A; Rebek Jr., J (2005). "A deep cavitand provides a structured environment for the menschutkin reaction". Journal of the American Chemical Society. 127 (32): 11222–11223. doi:10.1021/ja052877+. PMID 16089433.
- ↑ Subodh Kumar; Dharam Paul; Harjit Singh (2006). "Syntheses, structures and interactions of heterocalixarenes" (PDF). Arkivoc. 05-1699LU: 17–25.
- ↑ Geraci C, Consoli GM, Galante E, Bousquet E, Pappalardo M, Spadaro A (2008). "Calix[4]arene Decorated with Four Tn Antigen Glycomimetic Units and P3CS Immunoadjuvant: Synthesis, Characterization, and Anticancer Immunological Evaluation". Bioconjugate Chemistry. 19 (3): 751–758. doi:10.1021/bc700411w. PMID 18293897.
- ↑ Tatum, Prima R. (2009). The study of methods to synthesize silylated calixarenes in the 1,3-alternate conformation. ISBN 978-1-109-07989-0.