AP5
AP5
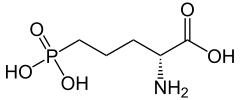 |
| Names |
| IUPAC name
2-amino-5-phosphonopentanoic acid |
| Identifiers |
| |
76326-31-3  N N |
| 3D model (Jmol) |
Interactive image |
| ChemSpider |
119225  Y Y |
| ECHA InfoCard |
100.150.904 |
| PubChem |
135342 |
InChI=1S/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11)/t4-/m1/s1  Y YKey: VOROEQBFPPIACJ-SCSAIBSYSA-N  Y YInChI=1/C5H12NO5P/c6-4(5(7)8)2-1-3-12(9,10)11/h4H,1-3,6H2,(H,7,8)(H2,9,10,11)/t4-/m1/s1 Key: VOROEQBFPPIACJ-SCSAIBSYBE
|
O=P(O)(O)CCC[C@@H](N)C(=O)O
|
| Properties |
| |
C5H12NO5P |
| Molar mass |
197.13 g/mol |
| Appearance |
white solid |
| Density |
1.529 g/mL |
| Boiling point |
482.1 °C (899.8 °F; 755.2 K) |
| |
Ammonium hydroxide, 50 mg/mL |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?) |
| Infobox references |
|
|
AP5 or APV ((2R)-amino-5-phosphonovaleric acid; (2R)-amino-5-phosphonopentanoate) is a selective NMDA receptor antagonist that competitively inhibits the ligand (glutamate) binding site of NMDA receptors.[1] AP5 blocks NMDA receptors in micromolar concentrations (~50 µM).
AP5 blocks the cellular analog of classical conditioning in the sea slug Aplysia californica, and has similar effects on Aplysia long-term potentiation (LTP), since NMDA receptors are required for both. It is sometimes used in conjunction with the calcium chelator BAPTA to determine whether NMDARs are required for a particular cellular process. AP5/APV has also been used to study NMDAR-dependent LTP in the mammalian hippocampus.[2]
In general, AP5 is very fast-acting within in vitro preparations, and can block NMDA receptor action at a reasonably small concentration. The active isomer of AP5 is considered to be the D configuration, although many preparations are available as a racemic mixture of D- and L-isomers. It is useful to isolate the action of other glutamate receptors in the brain, i.e., AMPA and kainate receptors.
AP5 can block the conversion of a silent synapse to an active one, since this conversion is NMDA receptor-dependent.
AP5 was developed by Jeff Watkins and Harry Olverman.
See also
References
- ↑ Morris RG. Synaptic plasticity and learning: selective impairment of learning rats and blockade of long-term potentiation in vivo by the N-methyl-D-aspartate receptor antagonist AP5. Journal of Neuroscience. 1989 Sep;9(9):3040-57. PMID 2552039
- ↑ Gustafsson B., Wigström H., Abraham W.C., and Huang Y.Y. Long-Term Potentiation in the Hippocampus Using Depolarizing Current Pulses as the Conditioning Stimulus to Single Volley Synaptic Potentials. Journal of Neuroscience. 1987 March;7(3):774-780
^ Laube, B; Hirai H, Sturgess M, Betz H, and Kuhse J (1997). "Molecular determinants of antagonists discrimination by NMDA receptor subunits: Analysis of the glutamate binding site on the NR2B subunit". Neuron 18 (3): 493–503. doi:10.1016/S0896-6273(00)81249-0. PMID 9115742.
|
|---|
|
Receptor
(ligands) | | AMPA | |
|---|
| | NMDA |
- Antagonists: Competitive antagonists: AP5 (APV)
- AP7
- CGP-37849
- CGP-39551
- CGP-39653
- CGP-40116
- CGS-19755
- CPP
- LY-233,053
- LY-235,959
- LY-274,614
- MDL-100,453
- Midafotel (d-CPPene)
- NPC-12,626
- NPC-17,742
- PBPD
- PEAQX
- Perzinfotel
- PPDA
- SDZ-220581
- Selfotel; Noncompetitive antagonists: ARR-15,896
- Caroverine
- Dexanabinol
- FPL-12495
- FR-115,427
- Hodgkinsine
- Magnesium
- MDL-27,266
- NPS-1506
- Psychotridine
- Zinc; Uncompetitive pore blockers: 2-MDP
- 3-HO-PCP
- 3-MeO-PCE
- 3-MeO-PCMo
- 3-MeO-PCP
- 4-MeO-PCP
- 8A-PDHQ
- 18-MC
- α-Endopsychosin
- Alaproclate
- Amantadine
- Aptiganel
- Arketamine
- ARL-12,495
- ARL-15,896-AR
- ARL-16,247
- Budipine
- Conaridine
- Delucemine
- Dexoxadrol
- Dextrallorphan
- Dieticyclidine
- Diphenidine
- Dizocilpine
- Ephenidine
- Esketamine
- Etoxadrol
- Eticyclidine
- Fluorolintane
- Gacyclidine
- Ibogaine
- Ibogamine
- Indantadol
- Ketamine
- Ketobemidone
- Lanicemine
- Loperamide
- Memantine
- Methadone (Levomethadone)
- Methorphan (Dextromethorphan
- Levomethorphan)
- Methoxetamine
- Methoxphenidine
- Milnacipran
- Morphanol (Dextrorphan
- Levorphanol)
- NEFA
- Neramexane
- Nitromemantine
- Nitrous oxide
- Noribogaine
- Norketamine
- Orphenadrine
- PCPr
- Pethidine (meperidine)
- Phencyclamine
- Phencyclidine
- Propoxyphene
- Remacemide
- Rhynchophylline
- Rimantadine
- Rolicyclidine
- Sabeluzole
- Tabernanthine
- Tenocyclidine
- Tiletamine
- Tramadol
- Xenon; Glycine site antagonists: 4-Cl-KYN (AV-101)
- 5,7-DCKA
- 7-CKA
- ACC
- ACEA-1011
- ACEA-1328
- AV-101
- Carisoprodol
- CGP-39653
- CNQX
- DNQX
- Felbamate
- Gavestinel
- GV-196,771
- Kynurenic acid
- Kynurenine
- L-689,560
- L-701,324
- Licostinel (ACEA-1021)
- LU-73,068
- MDL-105,519
- Meprobamate
- MRZ 2/576
- PNQX
- ZD-9379; NR2B subunit antagonists: Besonprodil
- CERC-301 (MK-0657)
- CO-101,244 (PD-174,494)
- Eliprodil
- Haloperidol
- Ifenprodil
- Isoxsuprine
- Nylidrin
- Ro8-4304
- Ro25-6981
- Traxoprodil; Polyamine site antagonists: Arcaine
- Co 101676
- Diaminopropane
- Diethylenetriamine
- Huperzine A
- Putrescine
- Ro 25-6981; Unclassified/unsorted antagonists: Bumetanide
- Chloroform
- Cyclopropane
- D-αAA
- Diethyl ether
- Enflurane
- Ethanol
- Flufenamic acid
- Flupirtine
- Furosemide
- Halothane
- Isoflurane
- Metaphit
- Methoxyflurane
- Niflumic acid
- Pentamidine isethionate
- Piretanide
- Toluene
- Transcrocetin (saffron)
- Trichloroethane
- Trichloroethanol
- Trichloroethylene
- Xylene
|
|---|
| | Kainate | |
|---|
| | mGlu1 | |
|---|
| | mGlu2 | |
|---|
| | mGlu3 | |
|---|
| | mGlu4 |
- Antagonists: CPPG
- MAP4
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
| | mGlu5 | |
|---|
| | mGlu6 |
- Antagonists: CPPG
- MAP4
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
| | mGlu7 |
- Antagonists: CPPG
- MAP4
- MMPIP
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
| | mGlu8 |
- Antagonists: CPPG
- MAP4
- MPPG
- MSOP
- MTPG
- UBP-1112
|
|---|
|
|---|
|
Transporter
(blockers) | |
|---|
|
Enzyme
(inhibitors) | |
|---|
|
| Others | |
|---|
|
See also: GABAergics • GHBergics • Glycinergics |
