Ryanodine receptor
| RyR domain | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| Symbol | RyR | ||||||||
| Pfam | PF02026 | ||||||||
| InterPro | IPR003032 | ||||||||
| TCDB | 1.A.3 | ||||||||
| OPM superfamily | 8 | ||||||||
| OPM protein | 5gl0 | ||||||||
| |||||||||
Ryanodine receptors (RyRs) form a class of intracellular calcium channels in various forms of excitable animal tissue like muscles and neurons.[1] There are three major isoforms of the ryanodine receptor, which are found in different tissues and participate in different signaling pathways involving calcium release from intracellular organelles. The RYR2 ryanodine receptor isoform is the major cellular mediator of calcium-induced calcium release (CICR) in animal cells.
Etymology
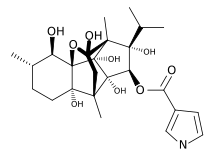
The ryanodine receptors are named after the plant alkaloid ryanodine, to which they show a high affinity:
Isoforms
There are multiple isoforms of ryanodine receptors:
- RyR1 is primarily expressed in skeletal muscle
- RyR2 is primarily expressed in myocardium (heart muscle)
- RyR3 is expressed more widely, but especially in the brain.[2]
- Non-mammalian vertebrates typically express two RyR isoforms, referred to as RyR-alpha and RyR-beta.
- Many invertebrates, including the model organisms Drosophila melanogaster (fruitfly) and Caenorhabditis elegans only have a single isoform. In non-metazoan species, calcium-release channels with sequence homology to RyRs can be found, but they are shorter than the mammalian ones and may be closer to IP3 Receptors.
|
|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Physiology
Ryanodine receptors mediate the release of calcium ions from the sarcoplasmic reticulum and endoplasmic reticulum, an essential step in muscle contraction.[1] In skeletal muscle, activation of ryanodine receptors occurs via a physical coupling to the dihydropyridine receptor (a voltage dependent L-type calcium channel), whereas, in cardiac muscle, the primary mechanism of activation is calcium-induced calcium release, which causes calcium outflow from the sarcoplasmic reticulum.[3]
It has been shown that calcium release from a number of ryanodine receptors in a ryanodine receptor cluster results in a spatiotemporally restricted rise in cytosolic calcium that can be visualised as a calcium spark.[4] Ryanodine receptors are very close to mitochondria and calcium release from RyR has been shown to regulate ATP production in heart and pancreas cells.[5][6][7]
Ryanodine receptors are similar to the inositol trisphosphate (IP3) receptor, and stimulated to transport Ca2+ into the cytosol by recognizing Ca2+ on its cytosolic side, thus establishing a positive feedback mechanism; a small amount of Ca2+ in the cytosol near the receptor will cause it to release even more Ca2+ (calcium-induced calcium release/CICR).[1]
RyRs are especially important in neurons and muscle cells. In heart and pancreas cells, another second messenger (cyclic ADP-ribose) takes part in the receptor activation.
The localized and time-limited activity of Ca2+ in the cytosol is also called a Ca2+ wave. The building of the wave is done by
- the feedback mechanism of the ryanodine receptor
- the activation of phospholipase C by GPCR or RTK, which leads to the production of inositol trisphosphate, which in turn activates the InsP3 receptor.
Associated proteins
RyRs form docking platforms for a multitude of proteins and small molecule ligands.[1] The cardiac-specific isoform of the receptor (RyR2) is known to form a quaternary complex with luminal calsequestrin, junctin, and triadin.[8] Calsequestrin has multiple Ca2+ binding sites and binds Ca2+ ions with very low affinity so they can be easily released.
Pharmacology
- Antagonists:[9]
- Ryanodine locks the RyRs at half-open state at nanomolar concentrations, yet fully closes them at micromolar concentration.
- Dantrolene the clinically used antagonist
- Ruthenium red
- procaine, tetracaine, etc. (local anesthetics)
- Activators:[10]
- Agonist: 4-chloro-m-cresol and suramin are direct agonists, i.e., direct activators.
- Xanthines like caffeine and pentifylline activate it by potentiating sensitivity to native ligand Ca.
- Physiological agonist: Cyclic ADP-ribose can act as a physiological gating agent. It has been suggested that it may act by making FKBP12.6 (12.6 kilodalton FK506 binding protein, as opposed to 12 kDa FKBP12 which binds to RyR1) which normally bind (and blocks) RyR2 channel tetramer in an average stoichiometry of 3.6, to fall off RyR2 (which is the predominant RyR in pancreatic beta cells, cardiomyocytes and smooth muscles).[11]
A variety of other molecules may interact with and regulate ryanodine receptor. For example: dimerized Homer physical tether linking inositol trisphosphate receptors (IP3R) and ryanodine receptors on the intracellular calcium stores with cell surface group 1 metabotropic glutamate receptors and the Alpha-1D adrenergic receptor[12]
Ryanodine
The plant alkaloid ryanodine, for which this receptor was named, has become an invaluable investigative tool. It can block the phasic release of calcium, but at low doses may not block the tonic cumulative calcium release. The binding of ryanodine to RyRs is use-dependent, that is the channels have to be in the activated state. At low (<10 MicroMolar, works even at nanomolar) concentrations, ryanodine binding locks the RyRs into a long-lived subconductance (half-open) state and eventually depletes the store, while higher (~100 MicroMolar) concentrations irreversibly inhibit channel-opening.
Caffeine
RyRs are activated by millimolar caffeine concentrations. High (greater than 5 mmol/L) caffeine concentrations cause a pronounced increase (from micromolar to picomolar) in the sensitivity of RyRs to Ca2+ in the presence of caffeine, such that basal Ca2+ concentrations become activatory. At low millimolar caffeine concentrations, the receptor opens in a quantal way, but has complicated behavior in terms of repeated use of caffeine or dependence on cytosolic or luminal calcium concentrations.
Role in disease
RyR1 mutations are associated with malignant hyperthermia and central core disease. RyR2 mutations play a role in stress-induced polymorphic ventricular tachycardia (a form of cardiac arrhythmia) and ARVD.[2] It has also been shown that levels of type RyR3 are greatly increased in PC12 cells overexpressing mutant human Presenilin 1, and in brain tissue in knockin mice that express mutant Presenilin 1 at normal levels, and thus may play a role in the pathogenesis of neurodegenerative diseases, like Alzheimer's disease.
The presence of antibodies against ryanodine receptors in blood serum has also been associated with myasthenia gravis.[1]
Structure
RyR1 cryo-EM structure revealed a large cytosolic assembly built on an extended α-solenoid scaffold connecting key regulatory domains to the pore. The RyR1 pore architecture shares the general structure of the six-transmembrane ion channel superfamily. A unique domain inserted between the second and third transmembrane helices interacts intimately with paired EF-hands originating from the α-solenoid scaffold, suggesting a mechanism for channel gating by Ca2+.[1][13]
See also
- Ryanoid, a class of insecticide that act through ryanodine receptors
References
- 1 2 3 4 5 6 Santulli, Gaetano; Marks, Andrew (2015). "Essential Roles of Intracellular Calcium Release Channels in Muscle, Brain, Metabolism, and Aging". Current Molecular Pharmacology. 8 (2): 206–222. doi:10.2174/1874467208666150507105105. ISSN 1874-4672.
- 1 2 Zucchi R, Ronca-Testoni S (March 1997). "The sarcoplasmic reticulum Ca2+ channel/ryanodine receptor: modulation by endogenous effectors, drugs and disease states". Pharmacol. Rev. 49 (1): 1–51. PMID 9085308.
- ↑ Fabiato A (1983). "Calcium-induced calcium release of calcium from the cardiac sarcoplasmic reticulum". Am J Physiol. 245 (1): C1–C14. PMID 6346892.
- ↑ Cheng H, Lederer WJ, Cannell MB (1993). "Calcium sparks: elementary events underlying excitation-contraction coupling in heart muscle". Science. 262 (5134): 740–744. doi:10.1126/science.8235594. PMID 8235594.
- ↑ Bround MJ, Wambolt R, Luciani DS, Kulpa JE, Rodrigues B, Brownsey RW, Allard MF, Johnson JD (May 2013). "Cardiomyocyte ATP production, metabolic flexibility, and survival require calcium flux through cardiac ryanodine receptors in vivo". J. Biol. Chem. 288 (26): 18975–86. doi:10.1074/jbc.M112.427062. PMID 23678000.
- ↑ Tsuboi T, da Silva Xavier G, Holz GG, Jouaville LS, Thomas AP, Rutter GA (January 2003). "Glucagon-like peptide-1 mobilizes intracellular Ca2+ and stimulates mitochondrial ATP synthesis in pancreatic MIN6 beta-cells". Biochem. J. 369 (Pt 2): 287–99. doi:10.1042/BJ20021288. PMC 1223096
 . PMID 12410638.
. PMID 12410638. - ↑ Dror V, Kalynyak TB, Bychkivska Y, Frey MH, Tee M, Jeffrey KD, Nguyen V, Luciani DS, Johnson JD (April 2008). "Glucose and endoplasmic reticulum calcium channels regulate HIF-1beta via presenilin in pancreatic beta-cells". J. Biol. Chem. 283 (15): 9909–16. doi:10.1074/jbc.M710601200. PMID 18174159.
- ↑ Kranias, Evangelia. "Dr. Evangelia Kranias Lab: Calsequestrin". Retrieved 22 May 2014.
- ↑ Vites AM, Pappano AJ (1994). "Distinct modes of inhibition by ruthenium red and ryanodine of calcium-induced calcium release in avian atrium". J Pharmacol Exp Ther. 268 (3): 1476–84. PMID 7511166.
- ↑ Xu L, Tripathy A, Pasek DA, Meissner G (1998). "Potential for pharmacology of ryanodine receptor/calcium release channels". Ann N Y Acad Sci. 853: 130–48. doi:10.1111/j.1749-6632.1998.tb08262.x. PMID 10603942.
- ↑ Wang YX, Zheng YM, Mei QB, Wang QS, Collier ML, Fleischer S, Xin HB, Kotlikoff MI (2004). "FKBP12.6 and cADPR regulation of Ca2+ release in smooth muscle cells". Am J Physiol Cell Physiol. 286 (3): C538–46. doi:10.1152/ajpcell.00106.2003. PMID 14592808.
- ↑ Tu JC, Xiao B, Yuan JP, Lanahan AA, Leoffert K, Li M, Linden DJ, Worley PF (1998). "Homer binds a novel proline-rich motif and links group 1 metabotropic glutamate receptors with IP3 receptors". Neuron. 21 (4): 717–26. doi:10.1016/S0896-6273(00)80589-9. PMID 9808459.
- ↑ Zalk R, Clarke OB, des Georges A, Grassucci RA, Reiken S, Mancia F, Hendrickson WA, Frank J, Marks AR (1 December 2014). "Structure of a mammalian ryanodine receptor.". Nature. Online first. doi:10.1038/nature13950. PMID 25470061.
External links
- Ryanodine Receptor at the US National Library of Medicine Medical Subject Headings (MeSH)