Polyoxometalate
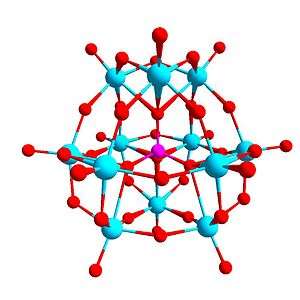
In chemistry, a polyoxometalate (abbreviated POM) is a polyatomic ion, usually an anion, that consists of three or more transition metal oxyanions linked together by shared oxygen atoms to form closed 3-dimensional frameworks. The metal atoms are usually group 6 (Mo, W) or less commonly group 5 (V, Nb, Ta) transition metals in their high oxidation states. They are usually colorless or orange, diamagnetic anions. Two broad families are recognized, isopolymetalates, composed of only one kind of metal and oxide, and heteropolymetalates, composed of one metal, oxide, and a main group oxyanion (phosphate, silicate, etc). Many exceptions to these general statements exist.
Formation
The oxides of d0 metals such as V2O5, MoO3, WO3 dissolve at high pH to give orthometalates, VO3−
4, MoO2−
4, WO2−
4. For Nb2O5 and Ta2O5, the nature of the dissolved species is less clear. As the pH is lowered, these orthometalates protonate to give oxide–hydroxide compounds such as W(OH)O−
3 and V(OH)O2−
3. These species condense via the process called olation. Condensation proceeds via loss of water and the formation of M–O–M linkages. An abbreviated condensation sequence illustrated with vanadates is:[1]
- 4 VO3−
4 + 8 H+ → V
4O4−
12 + 4 H2O - 2 1⁄2 V
4O4−
12 + 6 H+ → V
10O
26(OH)4−
2 + 2 H2O
When such acidifications are conducted in the presence of phosphate or silicate, then one obtains a heteropolymetalate. For example, the phosphotungstate anion PW
12O3−
40 consists of a framework of twelve octahedral tungsten oxyanions surrounding a central phosphate group.
History
The first example of a polyoxometalate compound was ammonium phosphomolybdate, containing the PMo
12O3−
40 anion, discovered in 1826.[2] This anion has the same structure as the phosphotungstate anion, whose structure was reported in 1934. This structure is called the Keggin structure after its discoverer.[3] Following this discovery, other fundamental structures such as the Wells–Dawson ion were found, and their chemistry and applications as catalysts were determined.
Structure
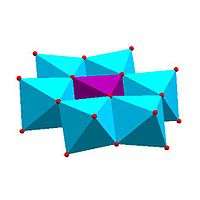
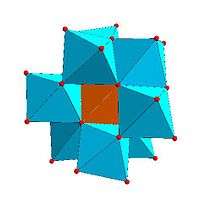
Certain structural motifs recur. The Keggin ion for example is common to both molybdates and tungstates with different central heteroatoms. Examples of some fundamental polyoxometalate structures are shown below. The Lindqvist ion and the rest of the first raw structures in the following figure are iso-polyoxometalates (isopolyanions in this case), since only one type of transition metal atoms is involved in their composition. The other structures are of the hetero-polyoxometalate type (heteropolyanions) since they involve more than one type of metal atom. The Keggin and Dawson structures have tetrahedrally-coordinated heteroatoms, such as P or Si, and the Anderson structure has an octahedral central atom, such as aluminium.
 |
 |
 |
 |
| Lindqvist hexamolybdate, Mo 6O2− 19 |
Decavanadate, V 10O6− 28 |
Paratungstate B, H 2W 12O10− 42 |
Mo36-polymolybdate, Mo 36O 112(H 2O)8− 16 |
 |
 |
 | |
| Strandberg structure, HP 2Mo 5O4− 23 |
Keggin structure, XM 12On− 40 |
Dawson structure, X 2M 18On− 62 | |
 |
 |
 |
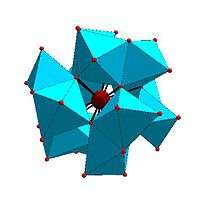 |
| Anderson structure, XM 6On− 24 |
Allman–Waugh structure, XM 9On− 32 |
Weakley–Yamase structure, XM 10On− 36 |
Dexter–Silverton structure, XM 12On− 42 |
Polyoxomolybdates include the wheel-shaped molybdenum blue anions and spherical keplerates. Numerous hybrid organic–inorganic materials that contain POM cores,[4][5] new potential applications based on unusual magnetic[6] and optical[7] properties of some POMs, and potential medical applications such as antitumor and antiviral uses.
Framework
The typical framework building blocks are polyhedral units, with 4-, 5-, 6- or 7-coordinate metal centres. These units share edges and/or vertices, or, less commonly, faces (such as in the ion CeMo
12O8−
42, which has face-shared octahedra with Mo atoms at the vertices of an icosahedron).[8]
The most common unit for polymolybdates is the octahedral {MoO6} unit, often distorted by the Mo atom being off-centre to give one shorter Mo–O bond. Some polymolybdates contain pentagonal bipyramidal units; these are the key building blocks in the molybdenum blues.
Heteroatoms

Heteroatoms are present in many polyoxometalates. Many different elements can serve as heteroatoms, with various coordination numbers:
- 4-coordinate (tetrahedral) in the Keggin, Dawson, and Lindqvist structures (e.g., PO3−
4, SiO4−
4, AsO3−
4) - 6-coordinate (octahedral) in the Anderson structure (e.g., Al(OH)3−
6, TeO6−
6) - 8-coordinate (square antiprismatic) in (CeO
8)W
10O8−
28 - 12-coordinate (icosahedral) in (UO
12)Mo
12O8−
30
The heteroatom may be located in the centre of the anion, as in the Keggin structure, or in the center of a structural fragment, such as the two phosphorus atoms in the Dawson ion, which are central to its two symmetric fragments.
Polyoxometalates bear similarities to clathrate structures. The Keggin ion, for example, could be formulated as PO3−
4@M
12O
36, and the Dawson as (XO2−
4)
2@M
18O
54. The @ notation denotes the physical enclosure of the left-hand side in the right-hand side. Unlike clathrates however, the guest anions cannot be reversibly removed.
Some cage structures that contain other ions are known. For example, the vanadate cage V18O42 can enclose a Cl− ion.[9] This structure has 5-coordinate square-pyramidal vanadium units linked together.
Isomerism
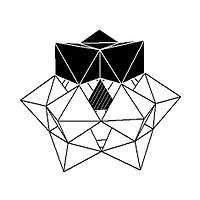
Isomerism is observed in some POMs. For example, the Keggin structure has 5 isomers, which are obtained by (conceptually) rotating one or more of the four {M3O13} units through 60°.
| α-XM 12On− 40 |
β-XM 12On− 40 |
γ-XM 12On− 40 |
δ-XM 12On− 40 |
ε-XM 12On− 40 |
|---|---|---|---|---|
 |
 |
 |
 |
 |
Lacunary structures
The structure of some POMs are derived from a larger POM's structure by removing one or more addenda atoms and their attendant oxide ions, giving a defective structure called a lacunary structure. An example of a compound with a Dawson lacunary structure is As2W15O56.[10] In 2014, vanadate species with similar, selective metal-binding properties were reported.[11]
Polyoxotantalates, niobates, and vanadates
The properties of polyniobates and polytantatalates are similar, but substantially different from the polyoxovanadates. In fact, polyvanadates are more similar to the oxomolybdates and tungstates.[12]
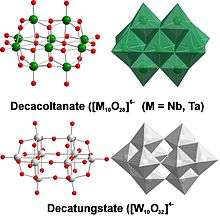
The similarities between the polyniobates and polytantatalates arise primarily from the equivalence in the charge of their stable 5+ ion and their size (64 pm) due to the lanthanide contraction. The most common members are M
6O8−
19 (M = Nb, Ta), which adopt the Lindqvist structure. These octaanions form in strongly basic conditions from alkali melts of the extended metal oxides (M2O5), or in the case of Nb even from mixtures of niobic acid and alkali metal hydroxides in aqueous solution. The hexatantalate can also be prepared by condensation of peroxotantalate Ta(O
2)3−
4 in alkaline media.[13] These polyoxometalates display an anomalous aqueous solubility trend of their alkali metal salts inasmuch as their Cs+ and Rb+ salts are more soluble than their Na+ and Li+ salts. The opposite trend is observed in group 6 POMs.[14] The decametalates with the formula M
10O6−
28 (M = Nb,[15] Ta[16]) are isostructural with decavanadate and are formed exclusively by edge-sharing {MO6} octahedra whereas the structure of decatungstate W
10O4−
32 comprises edge-sharing and corner-sharing tungstate octahedra.
Oxoalkoxometalates
Oxoalkoxometalates are clusters that contain both oxide and alkoxide ligands.[17] Typically they lack terminal oxo ligands. Examples include the dodecatitanate Ti12O16(OPri)16 (where OPri stands for an alkoxy group),[18] the iron oxoalkoxometalates[19] and iron Keggin ions.[20]
Sulfido, imido, and other O-replaced oxometalates
The terminal oxide centers of polyoxometalate framework can in certain cases be replaced with other ligands, such as S2−, Br−, and NR2−.[2][21] Sulfur-substituted POMs are called polyoxothiometalates. Other ligands replacing the oxide ions have also been demonstrated, such as nitrosyl and alkoxy groups.[17][22]
Applications
POMs are employed as commercial catalysts for oxidation of organic compounds.[23][24]
Potential and emerging applications
The range of size, structure, and elemental composition of polyoxometalates leads to a wide range of properties and a corresponding wide range of potential applications. Some of the applications include the following:
- "Green" oxidation catalysts as alternatives to chlorine-based wood pulp bleaching processes,[25] a method of decontaminating water,[26] and a method to catalytically produce formic acid from biomass (OxFA process).[27] Polyoxometalates have been shown to catalyse water splitting.[28]
- non-volatile (permanent) storage components, also known as flash memory devices.[29][30] Some POMs exhibit unusual magnetic properties[31] and are being investigated as possible nanocomputer storage devices (see qubits).[32]
- The catalytic epoxidation of olefins by using modified silver polyoxometalate catalyst (Ag/Ag-POM) and gold catalyst supported on the barium salt of the POM (2% Au/BaPOM) is very important in the chemical industry since epoxides are versatile and important intermediates in the synthesis of many fine chemicals and pharmaceuticals.
Biomedical research
Potential antitumor and antiviral drugs.[33] The Anderson-type polyoxomolybdates and heptamolybdates exhibit activity for suppressing the growth of some tumors. In the case of (NH3Pr)6[Mo7O24], activity appears related to its redox properties.[34][35]
POM with a Wells-Dawson structure can efficiently inhibit amyloid β (Aβ) aggregation in a therapeutic strategy for Alzheimer's Disease.[36]
References
- ↑ Greenwood, N. N.; Earnshaw, A. (1997). Chemistry of the Elements (2nd ed.). Oxford: Butterworth-Heinemann. ISBN 0-7506-3365-4.
- 1 2 Gouzerh, P.; Che, M. (2006). "From Scheele and Berzelius to Müller: polyoxometalates (POMs) revisited and the "missing link" between the bottom up and top down approaches". L’Actualité Chimique. 298: 9.
- ↑ Keggin, J. F. (1934). "The Structure and Formula of 12-Phosphotungstic Acid". Proc. Roy. Soc. A. 144 (851): 75–100. doi:10.1098/rspa.1934.0035.
- ↑ Song, Y.-F.; Long, D.-L.; Cronin, L. (2007). "Non covalently connected frameworks with nanoscale channels assembled from a tethered polyoxometalate–pyrene hybrid". Angew. Chem. Int. Ed. 46: 3900–3904. doi:10.1002/anie.200604734.
- ↑ Guo, Hong-Xu; Liu, Shi-Xiong (2004). "A novel 3D organic–inorganic hybrid based on sandwich-type cadmium heteropolymolybdate: [Cd4(H2O)2(2,2′-bpy)2] Cd[Mo6O12(OH)3(PO4)2(HPO4)2]2 [Mo2O4(2,2′-bpy)2]2·3H2O". Inorganic Chemistry Communications. 7 (11): 1217. doi:10.1016/j.inoche.2004.09.010.
- ↑ Müller, Achim; Luban, Marshall; Modler, Robert; Kögerler, Paul; Axenovich, Maria; Schnack, Jürgen; Canfield, Paul; Budko, Sergey; Harrison, Neil (2001). "Classical and Quantum Magnetism in Giant Keplerate Magnetic Molecules". ChemPhysChem. 2: 517. doi:10.1002/1439-7641(20010917)2:8/9<517::aid-cphc517>3.0.co;2-1.
- ↑ Schnack, Jürgen; Brüger, Mirko; Luban, Marshall; Kögerler, Paul; Morosan, Emilia; Fuchs, Ronald; Modler, Robert; Nojiri, Hiroyuki; Rai, Ram C.; Cao, Jinbo; Musfeldt, Janice; Wei, Xing (2006). "Field-dependent magnetic parameters in Ni4Mo12: Magnetostriction at the molecular level?". Phys. Rev. B. 73: 094401.
- ↑ Dexter, D. D.; Silverton, J. V. (1968). "A New Structural Type for Heteropoly Anions. The Crystal Structure of (NH4)2H6(CeMo12O42)·12H2O". J. Am. Chem. Soc. 1968: 3589.
- ↑ Müller, A.; Reuter, H.; Dillinger, S. (1995). "Supramolecular Inorganic Chemistry: Small Guests in Small and Large Hosts". Angew. Chem. Int. Ed. Engl. 34: 2328. doi:10.1002/anie.199523281.
- ↑ Mbombekalle, I. M.; Keita, B.; Nadjo, L.; Berthet, P.; Neiwert, W. A.; Hill, C. L.; Ritorto, M. D.; Anderson, T. M. (2003). "Manganous heteropolytungstates. Synthesis and heteroatom effects in Wells–Dawson-derived sandwich complexes". Dalton Trans. 2003: 2646–2650. doi:10.1039/b304255c.
- ↑ Kastner, K.; Margraf, J. T.; Clark, T.; Streb, C. (2014). "A Molecular Placeholder Strategy To Access a Family of Transition-Metal-Functionalized Vanadium Oxide Clusters". Chem. Eur. J. 20: 12269–12273. doi:10.1002/chem.201403592.
- ↑ "Hetero and lacunary polyoxovanadate chemistry: Synthesis, reactivity and structural aspects". Coord. Chem. Rev. 255: 2270–2280. 2011. doi:10.1016/j.ccr.2011.02.013.
- ↑ Fullmer, L. B.; Molina, P. I.; Antonio, M. R.; Nyman, M. (2014). Dalton Trans. 2014. doi:10.1039/C4DT02394C. Missing or empty
|title=(help) - ↑ Anderson, T. M.; Thoma, S. G.; Bonhomme, F.; Rodriguez, M. A.; Park, H.; Parise, J. B.; Alan, T. M.; Larentzos, J. P.; Nyman, M. (2007). Crystal Growth & Design. doi:10.1021/cg0606904. Missing or empty
|title=(help) - ↑ Graeber, E. J.; Morosin, B. (1977). Acta Crystallographica B. doi:10.1107/S0567740877007900. Missing or empty
|title=(help) - ↑ Matsumoto, M.; Ozawa, Y.; Yagasaki, A.; Zhe, Y. (2013). Inorg. Chem. doi:10.1021/ic400864e. Missing or empty
|title=(help) - 1 2 Pope, Michael Thor; Müller, Achim (1994). Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity. Springer. ISBN 0-7923-2421-8.
- ↑ Day, V. W.; Eberspacher, T. A.; Klemperer, W. G.; Park, C. W. (1993). "Dodecatitanates: a new family of stable polyoxotitanates". J. Am. Chem. Soc. 115: 8469–8470. doi:10.1021/ja00071a075.
- ↑ Bino, Avi; Ardon, Michael; Lee, Dongwhan; Spingler, Bernhard; Lippard, Stephen J. (2002). "Synthesis and Structure of [Fe13O4F24(OMe)12]5−: The First Open-Shell Keggin Ion". J. Am. Chem. Soc. 124: 4578–4579. doi:10.1021/ja025590a.
- ↑ Sadeghi, Omid; Zakharov, Lev N.; Nyman, May (2015). "Aqueous formation and manipulation of the iron-oxo Keggin ion". Science. 347: 1359–1362. doi:10.1126/science.aaa4620.
- ↑ Errington, R. John; Wingad, Richard L.; Clegg, William; Elsegood, Mark R. J. "Direct Bromination of Keggin Fragments To Give [PW9O28Br6]3−: A Polyoxotungstate with a Hexabrominated Face". Ang. Chem. 39 (21): 3884–3886. doi:10.1002/1521-3773(20001103)39:21<3884::AID-ANIE3884>3.0.CO;2-M.
- ↑ Gouzerh, P.; Jeannin, Y.; Proust, A.; Robert, F.; Roh, S.-G. (1993). "Functionalization of polyoxomolybdates: the example of nitrosyl derivatives". Mol. Eng. 3: 79. doi:10.1007/BF00999625.
- ↑ Misono, Makoto (1993). "Catalytic chemistry of solid polyoxometalates and their industrial applications". Mol. Eng. 3: 193–203. doi:10.1007/BF00999633.
- ↑ Kozhevnikov, Ivan V. (1998). "Catalysis by Heteropoly Acids and Multicomponent Polyoxometalates in Liquid-Phase Reactions". Chem. Rev. 98: 171–198. doi:10.1021/cr960400y.
- ↑ Gaspar, A. R.; Gamelas, J. A. F.; Evtuguin, D. V.; Neto, C. P. (2007). "Alternatives for lignocellulosic pulp delignification using polyoxometalates and oxygen: a review". Green Chem. 9: 717–730. doi:10.1039/b607824a.
- ↑ Hiskia, A.; Troupis, A.; Antonaraki, S.; Gkika, E.; Kormali, P.; Papaconstantinou, E. (2006). "Polyoxometallate photocatalysis for decontaminating the aquatic environment from organic and inorganic pollutants". Int. J. Env. Anal. Chem. 86 (3–4): 233. doi:10.1080/03067310500247520.
- ↑ Wölfel, R.; Taccardi, N.; Bösmann, A.; Wasserscheid, P. (2011). "Selective catalytic conversion of biobased carbohydrates to formic acid using molecular oxygen". Green Chem. 13: 2759. doi:10.1039/C1GC15434F.
- ↑ Rausch, B.; Symes, M. D.; Chisholm, G.; Cronin, L. (2014). "Decoupled catalytic hydrogen evolution from a molecular metal oxide redox mediator in water splitting". Science. 345: 1326–1330. doi:10.1126/science.1257443.
- ↑ "Flash memory breaches nanoscales", The Hindu.
- ↑ Busche, C.; Vila-Nadal, L.; Yan, J.; Miras, H. N.; Long, D.-L.; Georgiev, V. P.; Asenov, A.; Pedersen, R. H.; Gadegaard, N.; Mirza, M. M.; Paul, D. J.; Poblet, J. M.; Cronin, L. (2014). "Design and fabrication of memory devices based on nanoscale polyoxometalate clusters". Nature. 515: 545–549. doi:10.1038/nature13951.
- ↑ Müller, A.; Sessoli, R.; Krickemeyer, E.; Bögge, H; Meyer, J.; Gatteschi, D.; Pardi, L.; Westphal, J.; Hovemeier, K.; Rohlfing, R.; Döring, J; Hellweg, F.; Beugholt, C.; Schmidtmann, M. (1997). "Polyoxovanadates: High-Nuclearity Spin Clusters with Interesting Host-Guest Systems and Different Electron Populations. Synthesis, Spin Organization, Magnetochemistry, and Spectroscopic Studies". Inorg. Chem. 1997 (36): 5239–5240.
- ↑ Lehmann, J.; Gaita-Ariño, A.; Coronado, E.; Loss, D. (2007). "Spin qubits with electrically gated polyoxometalate molecules". Nanotechnology. 2: 312–317. doi:10.1038/nnano.2007.110.
- ↑ Rhule, Jeffrey T.; Hill, Craig L.; Judd, Deborah A. (1998). "Polyoxometalates in Medicine". Chem. Rev. 98: 327–358. doi:10.1021/cr960396q.
- ↑ Hasenknopf, Bernold,; Bernold; Hasenknopf. "Polyoxometalates: introduction to a class of inorganic compounds and their biomedical applications". Frontiers in Bioscience. 10 (1-3). doi:10.2741/1527.
- ↑ Pope, Michael; Müller, Achim. Polyoxometalates: From Platonic Solids to Anti-Retroviral Activity - Springer. pp. 337–342. doi:10.1007/978-94-011-0920-8.
- ↑ Gao, Nan; Sun, Hanjun; Dong, Kai; Ren, Jinsong; Duan, Taicheng; Xu, Can; Qu, Xiaogang (2014-03-04). "Transition-metal-substituted polyoxometalate derivatives as functional anti-amyloid agents for Alzheimer's disease". Nature Communications. 5: 3422. doi:10.1038/ncomms4422.
Further reading
- Long, D. L.; Burkholder, E.; Cronin, L. (2007). "Polyoxometalate clusters, nanostructures and materials: From self-assembly to designer materials and devices" (PDF). Chem. Soc. Rev. 36: 105–121. doi:10.1039/b502666k.
- Pope, M. T. (1983). Heteropoly and Isopoly Oxometalates. New York: Springer Verlag.
- Pope, M. T.; Müller, A. (1991). "Polyoxometalate Chemistry: An Old Field with New Dimensions in Several Disciplines". Angew. Chem. Int. Ed. Engl. 30: 34. doi:10.1002/anie.199100341.
- "Special volume on polyoxometalates". Chem. Rev. 98: 1. 1998.
- Cronin, L.; Müller, A., eds. (2012). "Special issue on polyoxometalates". Chem. Soc. Rev. 2012: 7325–7648.