Vanadate
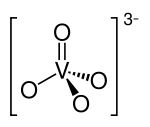
In chemistry, a vanadate is a compound containing an oxoanion of vanadium generally in its highest oxidation state of +5. The simplest vanadate ion is the tetrahedral, orthovanadate, VO3−
4 anion, which is present in e.g. sodium orthovanadate and in solutions of V2O5 in strong base (pH > 13 [1]). Conventionally this ion is represented with a single double bond, however this is a resonance form as the ion is a regular tetrahedron with four equivalent oxygen atoms.
Additionally a range of polyoxovanadate ions exist which include discrete ions and "infinite" polymeric ions.[2] There are also vanadates, such as rhodium vanadate, RhVO4, which has a statistical rutile structure where the Rh3+ and V5+ ions randomly occupy the Ti4+ positions in the rutile lattice,[3] that do not contain a lattice of cations and balancing vanadate anions but are mixed oxides.
In chemical nomenclature when vanadate forms part of the name, it indicates that the compound contains an anion with a central vanadium atom, e.g. ammonium hexafluorovanadate is a common name for the compound (NH4)3VF6 with the IUPAC name of ammonium hexafluoridovanadate(III).
Examples of vanadate ions
Some examples of discrete ions are
- VO3−
4 "orthovanadate", tetrahedral.[2] - V
2O4−
7 "pyrovanadate", corner-shared VO4 tetrahedra, similar to the dichromate ion[2] - V
3O3−
9, cyclic with corner-shared VO4 tetrahedra[4] - V
4O4−
12, cyclic with corner-shared VO4 tetrahedra[5] - V
5O3−
14, corner shared VO4 tetrahedra[6] - V
10O6−
28 "decavanadate", edge- and corner-shared VO6 octahedra[2] - V
12O4−
32[2] - V
13O3−
34, fused VO6 octahedra [7] - V
18O12−
42[8]
Some examples of polymeric “infinite” ions are
 |
 |
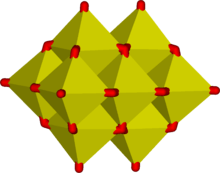 |
In these ions vanadium exhibits tetrahedral, square pyramidal and octahedral coordination. In this respect vanadium shows similarities to tungstate and molybdate, whereas chromium however has a more limited range of ions.
Aqueous solutions
Dissolution of vanadium pentoxide in strongly basic aqueous solution gives the colourless VO3−
4 ion. On acidification, this solution's colour gradually darkens through orange to red at around pH 7. Brown hydrated V2O5 precipitates around pH 2, redissolving to form a light yellow solution containing the [VO2(H2O)4]+ ion. The number and identity of the oxyanions that exist between pH 13 and 2 depend on pH as well as concentration. For example, protonation of vanadate initiates a series of condensations to produce polyoxovanadate ions:[2]
- pH 9–12; HVO2−
4, V
2O4−
7 - pH 4–9; H
2VO−
4, V
4O4−
12, HV
10O5−
28 - pH 2–4; H3VO4, H
2V
10O4−
28
Pharmacological properties
Vanadate is a potent inhibitor of certain plasma membrane ATPases, such as Na+/K+-ATPase and Ca2+-ATPase (PMCA). However, it does not inhibit other ATPases, such as SERCA (sarco/endoplasmic reticulum Ca2+-ATPase), actomyosin ATPase and mitochondrial ATPase.[10][11] Aureliano, Manuel; Crans, Debbie C. (2009). "Decavanadate and oxovanadates: Oxometalates with many biological activities". Journal Inorganic Biochemistry 103: 536–546. doi:10.1016/j.jinorgbio.2008.11010.
References
- ↑ Cotton, F. Albert; Wilkinson, Geoffrey; Murillo, Carlos A.; Bochmann, Manfred (1999), Advanced Inorganic Chemistry (6th ed.), New York: Wiley-Interscience, ISBN 0-471-19957-5
- 1 2 3 4 5 6 7 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 0-08-037941-9.
- ↑ Wells A.F. (1984) Structural Inorganic Chemistry 5th edition Oxford Science Publications ISBN 0-19-855370-6
- ↑ Hamilton E. E.; Fanwick P.E.; Wilker J.J. (2002). "The Elusive Vanadate (V3O9)3−: Isolation, Crystal Structure, and Nonaqueous Solution Behavior". J. Am. Chem. Soc. 124 (1): 78. doi:10.1021/ja010820r.
- ↑ G.-Y. Yang, D.-W. Gao, Y. Chen, J.-Q. Xu, Q.-X. Zeng, H.-R. Sun, Z.-W. Pei, Q. Su, Y. Xing, Y.-H. Ling and H.-Q. Jia (1998). "[Ni(C10H8N2)3]2[V4O12]·11H2O". Acta Crystallographica C. 54 (5): 616. doi:10.1107/S0108270197018751.
- ↑ V. W. Day; Walter G. Klemperer; O. M. Yaghi (1989). "A new structure type in polyoxoanion chemistry: synthesis and structure of the V
5O3−
14 anion". J. Am. Chem. Soc. 111 (12): 4518. doi:10.1021/ja00194a068. - ↑ Hou D.; Hagen K.D.; Hill C.L. (1992). "Tridecavanadate, [V13O34]3−, a new high-potential isopolyvanadate". J. Am. Chem. Soc. 114 (14): 5864. doi:10.1021/ja00040a061.
- ↑ Müller A.; Sessoli R.; Krickemeyer E.; Bögge H.; Meyer J.; Gatteschi D.; Pardi L.; Westphal J.; Hovemeier K.; Rohlfing R.; Döring J; Hellweg F.; Beugholt C.; Schmidtmann M. (1997). "Polyoxovanadates: High-Nuclearity Spin Clusters with Interesting Host-Guest Systems and Different Electron Populations. Synthesis, Spin Organization, Magnetochemistry, and Spectroscopic Studies". Inorg. Chem. 36 (23): 5239. doi:10.1021/ic9703641.
- ↑ Jouanneau, S.; Verbaere, A.; Guyomard, D. (2003). "On a new calcium vanadate: synthesis, structure and Li insertion behaviour". Journal of Solid State Chemistry. 172: 116. Bibcode:2003JSSCh.172..116J. doi:10.1016/S0022-4596(02)00164-0.
- ↑ Luo D.; Nakazawa M.; Yoshida Y.; Cai J.; Imai S. (2000). "Effects of three different Ca2+ pump ATPase inhibitors on evoked contractions in rabbit aorta and activities of Ca2+ pump ATPases in porcine aorta". General Pharmacology: The Vascular System. 34 (3): 211–220. doi:10.1016/S0306-3623(00)00064-1.
- ↑ Bowman B.J.; Slayman C.W. (1979). "The Effects of Vanadate on the Plasma Membrane ATPase of Neurospora crassa". Journal of Biological Chemistry. 254 (8): 2928–2934. PMID 155060.