Phenylpropanoid
The phenylpropanoids are a diverse family of organic compounds that are synthesized by plants from the amino acids phenylalanine and tyrosine.[1] Their name is derived from the six-carbon, aromatic phenyl group and the three-carbon propene tail of cinnamic acid, which is synthesized from phenylalanine in the first step of phenylpropanoid biosynthesis. Phenylpropanoids are found throughout the plant kingdom, where they serve as essential components of a number of structural polymers, provide protection from ultraviolet light, defend against herbivores and pathogens, and mediate plant-pollinator interactions as floral pigments and scent compounds. Concentrations of phenylpropanoids within plants are also altered by changes in resource availability.[2]
Phenylpropanoids and other phenolics are part of the chemical composition of sporopollenin. This substance found in pollen is not exactly known, due to its unusual chemical stability and resistance to degradation by enzymes and strong chemical reagents. Analyses have revealed a mixture of biopolymers, containing mainly long chain fatty acids, phenylpropanoids, phenolics and traces of carotenoids. Tracer experiments have shown that phenylalanine is a major precursor, but other carbon sources also contribute. It is likely that sporopollenin is derived from several precursors that are chemically cross-linked to form a rigid structure.
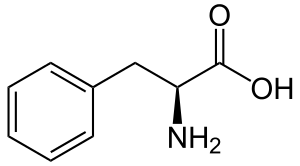

Hydroxycinnamic acids

Phenylalanine is first converte to cinnamic acid by the action of the enzyme phenylalanine ammonia-lyase (PAL). Some plants, mainly monocotyledonous, use tyrosine to synthesize p-coumaric acid by the action of the bifunctional enzyme Phenylalanine/tyrosine ammonia-lyase (PTAL). A series of enzymatic hydroxylations and methylations leads to coumaric acid, caffeic acid, ferulic acid, 5-hydroxyferulic acid, and sinapic acid. Conversion of these acids to their corresponding esters produces some of the volatile components of herb and flower fragrances, which serve many functions such as attracting pollinators. Ethyl cinnamate is a common example.
Cinnamic aldehydes and monolignols
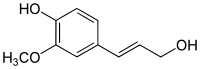
Reduction of the carboxylic acid functional groups in the cinnamic acids provides the corresponding aldehydes, such as cinnamaldehyde. Further reduction provides monolignols including coumaryl alcohol, coniferyl alcohol, and sinapyl alcohol, which vary only in their degree of methoxylation. The monolignols are monomers that are polymerized to generate various forms of lignin and suberin, which are used as a structural component of plant cell walls.
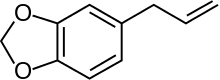
The phenylpropenes, including eugenol, chavicol, safrole and estragole, are also derived from the monolignols. These compounds are the primary constituents of various essential oils.
Coumarins and flavonoids

Hydroxylation of cinnamic acid in the 4-position by trans-cinnamate 4-monooxygenase leads to p-coumaric acid, which can be further modified into hydroxylated derivatives such as umbelliferone. Another use of p-coumaric acid via its thioester with coenzyme A, i.e. 4-coumaroyl-CoA, is the production of chalcones. This is achieved with the addition of 3 malonyl-CoA molecules and their cyclization into a second phenyl group. Chalcones are the precursors of all flavonoids, a diverse class of phytochemicals.
Stilbenoids
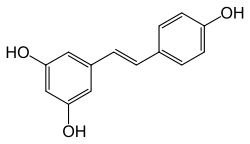
Stilbenoids, such as resveratrol, are hydroxylated derivatives of stilbene. They are formed through an alternative cyclization of cinnamoyl-CoA or 4-coumaroyl-CoA.
Roles in nature
In the orchid Phalaenopsis, phenylpropanoid enzymes (shikimate dehydrogenase, phenylalanine ammonia-lyase (PAL) and cinnamyl alcohol dehydrogenase (CAD)) are enhanced in the process of plant acclimatisation at different levels of photosynthetic photon flux.[3]
See also
References
- ↑ Barros J, Serrani-Yarce JC, Chen F, Baxter D, Venables BJ, Dixon RA. 2016. Role of bifunctional ammonia-lyase in grass cell wall biosynthesis. Nat. Plants. 2: 16050. doi:10.1038/nplants.2016.50. PMID 27255834
- ↑ Davey MP, DN Bryant, I Cummins, P Gates, TW Ashenden, R Baxter, R Edwards. 2004. Effects of elevated CO2 on the vasculature and phenolic secondary metabolism of Plantago maritima. Phytochemistry. 65. 2197-2204
- ↑ Enhancement of phenylpropanoid enzymes and lignin in Phalaenopsis orchid and their influence on plant acclimatisation at different levels of photosynthetic photon flux. Mohammad Babar Ali, Serida Khatun, Eun-Joo Hahn and Kee-Yoeup Paek, Plant Growth Regulation, 2006, Volume 49, Numbers 2-3, pages 137-146, doi:10.1007/s10725-006-9003-z
- K Hahlbrock, D Scheel (1989). "Physiology and Molecular Biology of Phenylpropanoid Metabolism". Annual Review of Plant Physiology and Plant Molecular Biology. 40: 347–69. doi:10.1146/annurev.pp.40.060189.002023.