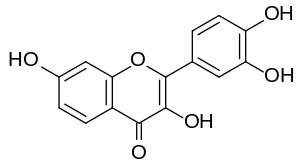
Fisetin
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-(3,4-dihydroxyphenyl)-3,7-dihydroxychromen-4-one | |
| Other names
Cotinin (not to be confused with Cotinine) 5-Deoxyquercetin Superfustel Fisetholz Fietin Fustel Fustet Viset Junger fustik | |
| Identifiers | |
| 528-48-3 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:42567 |
| ChEMBL | ChEMBL31574 |
| ChemSpider | 4444933 |
| DrugBank | DB07795 |
| ECHA InfoCard | 100.007.669 |
| 5182 | |
| KEGG | C10041 |
| PubChem | 5281614 |
| |
| |
| Properties | |
| C15H10O6 | |
| Molar mass | 286.2363 g/mol |
| Density | 1.688 g/mL |
| Melting point | 330 °C (626 °F; 603 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Fisetin (3, 7, 3′, 4′-tetrahydroxyflavone) is a flavonol, a structurally distinct chemical substance that belongs to the flavonoid group of polyphenols. It can be found in many plants, where it serves as a colouring agent. It is also found in many fruits and vegetables, such as strawberries, apples, persimmons, onions and cucumbers.[1] Its chemical formula was first described by Austrian chemist Josef Herzig in 1891.[2]
The biological activity of fisetin has been studied in many laboratory assays; like other polyphenols it has many activities.
Biological sources
Fisetin can be found in a wide variety of plants. It is found in Eudicotyledons, such as trees and shrubs in the family Fabaceae, such as the acacias Acacia greggii[3] and Acacia berlandieri,[3] the parrot tree (Butea frondosa), the honey locust (Gleditsia triacanthos), members of the family Anacardiaceae such as the Quebracho colorado and species of the genus Rhus, which contains the sumacs.[4] Along with myricetin, fisetin provides the color of the traditional yellow dye young fustic, which was extracted from the Eurasian smoketree (Rhus cotinus). Many fruits and vegetables also contain fisetin,[5] including strawberries[6][7] apples,[7] and grapes.[7][8] Fisetin can be extracted from fruit and herbal sources in juices, wines,[9] and infusions such as teas.[8] It is also found in Monocotyledons such as onions.[7] It is also present in Pinophyta species such as the yellow cypress (Callitropsis nootkatensis).
Biosynthesis
Fisetin is a polyphenol, which is a flavonoid subgroup. Flavonoid synthesis begins with the phenylpropanoid pathway, in which phenylalanine, an amino acid, is transformed into 4-coumaroyl-CoA. This is the compound that enters the flavonoid biosynthesis pathway. Chalcone synthase, the first enzyme of this pathway, produces chalcone from 4-coumaroyl-CoA. All flavonoids are derived from this chalcone backbone. The activity of different enzymes, including isomerases and hydroxylases, alter the backbone depending on the subclass of the flavonoid being produced. Transferases help control changes in the flavonoid’s solubility and reactivity by catalyzing the addition of things such as methyl groups and sugars. This allows for controlled fluctuations in physiological activities.[10]
Flavonoid biosynthesis gene regulation occurs through the interaction of different transcription factors. Depending on the combination of transcription factor interactions, the structural genes involved in flavonoid biosynthesis are expressed in specific locations of the plant and at specific times. Many myeloblastosis (MYB) transcription factors have been identified in a variety of fruits and plants, including strawberries, maize, and arabidopsis, as being important in the regulation of flavonoid biosynthesis and accumulation. These transcription factors continue to be studied in plant model organisms such as maize and Arabidopsis.[10]
The environment of the plant has also been shown to affect the flavonoid biosynthesis pathway. Shorter wavelengths of light, ranging from blue to UV light, allow for higher production and accumulation of flavonoids in fruits. These wavelengths activate enzymes that are involved in the phenylpropanoid and flavonoid biosynthesis pathways, stimulating the production of flavonoids. The level of stimulation can vary between individual fruits.[11]
Research
Fisetin, like other polyphenols such as resveratrol, is a sirtuin-activating compound and has been shown in laboratory studies to extend the life of simple organisms like yeast, worms, and flies.[12] Like the other compounds, it has also been shown to be reactive in many different assays of biological activities, raising the possibility that any drug generated from fisetin would have too many side effects to be useful.[12][13]
Fisetin has shown anti-cancer activity in studies on cells and model animals conducted in laboratories, and appears to block the PI3K/AKT/mTOR pathway.[14] In lab studies it also has been shown to be an anti-proliferative agent, interfering with the cell cycle in several ways.[15] Fisetin, like some other flavonoids, has been found in lab studies to be a topoisomerase inhibitor, which may turn out to be a carcinogenic activity or an anti-cancer activity - further research is needed.[16]
In studies conducted on cells in a laboratory, fisetin inhibits the activity of several pro-inflammatory cytokines, including tumor necrosis factor alpha, interleukin 6, and Nuclear factor kappa B.[15] It has also has been shown in lab studies to upregulate glutathione, an endogenous antioxidant.[15][17] Fisetin also has direct activity as a reducing agent, chemically reacting with reactive oxygen species to neutralize them.[17] Based on lab studies, it appears that fisetin lodges in cell membranes and prevents oxidative damage to lipids in the cell membrane.[17] Fisetin, like other flavonoids, has a planar structure, with multiple carbon rings. Fisetin is able to scavenge free radicals as a result of its electron donating capacity, which is due to the presence of two hydroxyl groups on one ring and a hydroxyl group on another ring.[17]
References
- ↑ Sahu, Bidya Dhar; Kalvala, Anil Kumar; Koneru, Meghana; Kumar, Jerald Mahesh; Kuncha, Madhusudana; Rachamalla, Shyam Sunder; Sistla, Ramakrishna (September 3, 2014). "Ameliorative Effect of Fisetin on Cisplatin-Induced Nephrotoxicity in Rats via Modulation of NF-κB Activation and Antioxidant Defence". PLOS ONE. 9 (9): e105070. doi:10.1371/journal.pone.0105070.
- ↑ Herzig, J. (1891). "Studien über Quercetin und seine Derivate, VII. Abhandlung" [Studies on Quercetin and its Derivatives, Treatise VII]. Monatshefte für Chemie (in German). 12 (1): 177–90. doi:10.1007/BF01538594.
- 1 2 Forbes TDA, Clement BA. "Chemistry of Acacia's from South Texas" (PDF). Texas A&M Agricultural Research and Extension Center at. Archived from the original (PDF) on May 15, 2011. Retrieved 2010-04-14.
- ↑ Gábor, M.; Eperjessy, E. (1966). "Antibacterial Effect of Fisetin and Fisetinidin". Nature. 212 (5067): 1273. doi:10.1038/2121273a0. PMID 21090477.
- ↑ Fiorani, M.; Accorsi, A. (2005). "Dietary flavonoids as intracellular substrates for an erythrocyte trans-plasma membrane oxidoreductase activity". The British journal of nutrition. 94 (3): 338–345. doi:10.1079/bjn20051504. PMID 16176603.
- ↑ Maher, Pamela; Dargusch, Richard; Ehren, Jennifer L.; Okada, Shinichi; Sharma, Kumar; Schubert, David (2011). Deli, Maria A., ed. "Fisetin Lowers Methylglyoxal Dependent Protein Glycation and Limits the Complications of Diabetes". PLoS ONE. 6 (6): e21226. doi:10.1371/journal.pone.0021226. PMC 3124487
 . PMID 21738623. Lay summary – ScienceDaily (June 28, 2011).
. PMID 21738623. Lay summary – ScienceDaily (June 28, 2011). - 1 2 3 4 Arai, Y.; Watanabe, S.; Kimira, M.; Shimoi, K.; Mochizuki, R.; Kinae, N. (2000). "Dietary intakes of flavonols, flavones and isoflavones by Japanese women and the inverse correlation between quercetin intake and plasma LDL cholesterol concentration". The Journal of Nutrition. 130 (9): 2243–2250. PMID 10958819.
- 1 2 Viñas, P.; Martínez-Castillo, N.; Campillo, N.; Hernández-Córdoba, M. (2011). "Directly suspended droplet microextraction with in injection-port derivatization coupled to gas chromatography–mass spectrometry for the analysis of polyphenols in herbal infusions, fruits and functional foods". Journal of Chromatography A. 1218 (5): 639–646. doi:10.1016/j.chroma.2010.12.026. PMID 21185565.
- ↑ De Santi, C.; Pietrabissa, A.; Mosca, F.; Pacifici, G. M. (2002). "Methylation of quercetin and fisetin, flavonoids widely distributed in edible vegetables, fruits and wine, by human liver". International journal of clinical pharmacology and therapeutics. 40 (5): 207–212. doi:10.5414/cpp40207. PMID 12051572.
- 1 2 Ferreyra, M.L.; Rius, S.P.; Casati, P. (September 28, 2012). "Flavanoids: biosynthesis, biological functions, and biotechnological applications". Frontiers in Plant Science. 3 (222). doi:10.3389/fpls.2012.00222. PMC 3460232
 . PMID 23060891.
. PMID 23060891. - ↑ Zoratti, L.; Karppinen, K.; Escobar, A.L.; Haggman, H.; Jaakola, L. (October 9, 2014). "Light-controlled flavanoid biosynthesis in fruits". Frontiers in Plant Science. 5 (534). doi:10.3389/fpls.2014.00534. PMC 4191440
 . PMID 25346743.
. PMID 25346743. - 1 2 Baur, JA (August 2010). "Biochemical effects of SIRT1 activators.". Biochimica et biophysica acta. 1804 (8): 1626–34. PMC 2886178
 . PMID 19897059.
. PMID 19897059. - ↑ "How should we assess the effects of exposure to dietary polyphenols in vitro?". Am. J. Clin. Nutr. 80 (1): 15–21. July 2004. PMID 15213022.
- ↑ Syed, DN; et al. (Sep 2013). "Inhibition of Akt/mTOR signaling by the dietary flavonoid fisetin". Anticancer Agents Med Chem. 13 (7): 995–1001. doi:10.2174/18715206113139990129. PMC 3985520
 . PMID 23293889.
. PMID 23293889. - 1 2 3 Gupta, SC; et al. (1 October 2014). "Downregulation of tumor necrosis factor and other proinflammatory biomarkers by polyphenols.". Archives of biochemistry and biophysics. 559: 91–9. PMID 24946050.
- ↑ "Recent advances in the development of dual topoisomerase I and II inhibitors as anticancer drugs.". Curr Med Chem. 17 (35): 4270–90. 2010. doi:10.2174/092986710793361252. PMID 20939813.
- 1 2 3 4 "Fisetin: a dietary antioxidant for health promotion.". Antioxidants. 19 (2): 151–62. Jul 2013. doi:10.1089/ars.2012.4901. PMC 3689181
 . PMID 23121441.
. PMID 23121441.