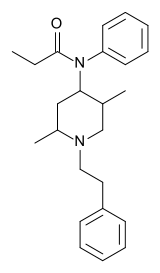
Phenaridine
 | |
| Clinical data | |
|---|---|
| ATC code | none |
| Identifiers | |
| |
| CAS Number | 42045-97-6 |
| PubChem (CID) | 162056 |
| ChemSpider |
142327 |
| Chemical and physical data | |
| Formula | C24H32N2O |
| Molar mass | 364.52 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Phenaridine (2,5-Dimethylfentanyl) is an opioid analgesic that is an analogue of fentanyl. It was developed in 1972,[1] and is used for surgical anasthesia.[2][3]
Phenaridine has similar effects to fentanyl. It is slightly less potent than fentanyl in rats.[1] Side effects of fentanyl analogs are similar to those of fentanyl itself, which include itching, nausea and potentially serious respiratory depression, which can be life-threatening. Fentanyl analogs have killed hundreds of people throughout Europe and the former Soviet republics since the most recent resurgence in use began in Estonia in the early 2000s, and novel derivatives continue to appear.[4]
See also
- 3-Methylfentanyl
- 4-Fluorofentanyl
- α-Methylfentanyl
- Acetylfentanyl
- Furanylfentanyl
- List of Fentanyl analogues
References
- 1 2 Riley NT, Hale DB, Wilson MV. 4-Anilidopiperidine Analgesics I: Synthesis and Analgesic Activity of Certain Ring-Methylated 1-Substituted 4-Propananilidopiperidines. J. Pharm. Sci 62(6): 983 (1973)
- ↑ Osipova NA, Petrova VV, Novikov GA, Dolgopolova TV, Zhukova OI, Mel'nikova ZL, Smolina TA. The new Soviet narcotic analgesic phenaridine as a component of general anesthesia during cancer surgery. (Russian). Anesteziologiia i Reanimatologiia. 1991 Jan-Feb;(1):42-6.
- ↑ Vlasenko EV, Durgarian LK, Azlivian AS, Sarukhanian KV. The evaluation of the analgesic action of phenaridine when combined with agents used in anesthesiological practice. (Russian). Farmakologiia i Toksikologiia. 1991 May-Jun;54(3):17-20.
- ↑ Jane Mounteney, Isabelle Giraudon, Gleb Denissov, Paul Griffiths (July 2015). "Fentanyls: Are we missing the signs? Highly potent and on the rise in Europe.". The international journal of drug policy. 26 (7): 626–631. doi:10.1016/j.drugpo.2015.04.003. PMID 25976511.
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.