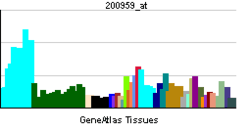
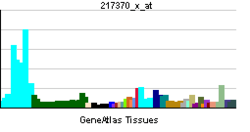
FUS (gene)
| View/Edit Human | |||
RNA-binding protein FUS/TLS (Fused in Sarcoma/Translocated in Sarcoma) is a protein that in humans is encoded by the FUS gene.[2][3][4][5][6][7]
Structure and function
The N-terminal end of FUS appears to be involved in transcriptional activation, while the C-terminal end is involved in protein and RNA binding. In addition recognition sites for the transcription factors AP2, GCF, Sp1 have been identified in FUS.[8]
FUS/TLS is a member of the TET protein family that also includes the EWS protein, the TATA-binding protein (TBP)-associated factor (TAFII68/TAF15) and the Drosophila cabeza/SARF protein.[9][10]
FUS/TLS, EWS and TAFII68/TAF15 have a similar structure characterised by an N-terminal QGSY-rich region, a highly conserved RNA recognition motif (RRM), multiple RGG repeats, which are extensively dimethylated at arginine residues[11] and a C-terminal zinc finger motif.[4][6][9][12]
Discovery
FUS/TLS was initially identified as a fusion protein (FUS-CHOP) caused by chromosomal translocations in human cancers especially liposarcomas.[3][6] In these instances, the promoter and N-terminal part of FUS/TLS is translocated to the C-terminal domain of various DNA-binding transcription factors (eg CHOP) conferring a strong transcriptional activation domain to the fusion proteins.[10][13]
FUS/TLS was independently identified as the hnRNP P2 protein, a subunit of a complex involved in maturation of pre-mRNA.[14]
Function
Consistently, in vitro studies have shown that FUS/TLS binds RNA, single-stranded DNA and (with lower affinity) double-stranded DNA.[4][6][15][16][17][18] The sequence specificity of FUS/TLS binding to RNA or DNA has not been well established; however, using in vitro selection (SELEX), a common GGUG motif has been identified in approximately half of the RNA sequences bound by FUS/TLS.[19] A later proposal was that the GGUG motif is recognised by the zinc finger domain and not the RRM (80). Additionally, FUS/TLS has been found to bind a relatively long region in the 3′ untranslated region (UTR) of the actin-stabilising protein Nd1-L mRNA, suggesting that rather than recognising specific short sequences, FUS/TLS interacts with multiple RNA-binding motifs or recognises secondary conformations.[20] FUS/TLS has also been proposed to bind human telomeric RNA (UUAGGG)4 and single-stranded human telomeric DNA in vitro.[21]
Beyond nucleic acid binding, FUS/TLS was also found to associate with both general and more specialized protein factors to influence the initiation of transcription.[22] Indeed, FUS/TLS interacts with several nuclear receptors.[23] and with gene-specific transcription factors such as Spi-1/PU.1.[24] or NF-κB.[25] It also associates with the general transcriptional machinery and may influence transcription initiation and promoter selection by interacting with RNA polymerase II and the TFIID complex.[26][27][28] Recently, FUS/TLS was also shown to repress the transcription of RNAP III genes and to co-immunoprecipitate with TBP and the TFIIIB complex.[29]
Clinical significance
FUS gene rearrangement has been implicated in the pathogenesis of both myxoid liposarcoma and low grade fibromyxoid sarcoma.
In 2009 two separate research groups analysed 26 unrelated families who presented with a type6 ALS phenotype, and found 14 mutations in the FUS gene.[30][31]
Subsequently, FUS has also emerged as a significant disease protein in a subgroup of frontotemporal lobar dementias (FTLDs), previously characterized by immunoreactivity of the neuronal inclusions for ubiquitin, but not for TDP-43 or tau with a proportion of the inclusions also containing a-internexin in a further subgroup known as neuronal intermediate filament inclusion disease (NIFID). The disease entities which are now considered subtypes of FTLD-FUS are atypical frontotemporal lobar degeneration with ubiquitinated inclusions (aFTLD-U), NIFID (otherwise known as neurofilament inclusion body disease) and basophilic inclusion body disease (BIBD), which together with ALS-FUS comprise the FUS-opathies.[32][33][34][35]
FTLD is the pathological term for the clinical syndrome of frontotemporal dementia (FTD). FTD differs from the more common Alzheimer's dementia in that memory is relatively well preserved, instead the disease presents with a more temporal-lobe phenotype. Behavioural variant frontotemporal dementia (bvFTD), progressive non-fluent aphasia (PNFA) and semantic dementia (SD) are the three best-characterised clinical presentations. FUS positive FTLD tends to present clinically as a bvFTD but the correlation between underlying pathology and clinical presentation is not perfect.
Interactions
FUS has been shown to interact with:
References
- ↑ "Human PubMed Reference:".
- ↑ Eneroth M, Mandahl N, Heim S, Willén H, Rydholm A, Alberts KA, Mitelman F (Aug 1990). "Localization of the chromosomal breakpoints of the t(12;16) in liposarcoma to subbands 12q13.3 and 16p11.2". Cancer Genet Cytogenet. 48 (1): 101–7. doi:10.1016/0165-4608(90)90222-V. PMID 2372777.
- 1 2 Rabbitts TH, Forster A, Larson R, Nathan P (Sep 1993). "Fusion of the dominant negative transcription regulator CHOP with a novel gene FUS by translocation t(12;16) in malignant liposarcoma". Nat Genet. 4 (2): 175–80. doi:10.1038/ng0693-175. PMID 7503811.
- 1 2 3 Prasad DD, Ouchida M, Lee L, Rao VN, Reddy ES (December 1994). "TLS/FUS fusion domain of TLS/FUS-erg chimeric protein resulting from the t(16;21) chromosomal translocation in human myeloid leukemia functions as a transcriptional activation domain". Oncogene. 9 (12): 3717–29. PMID 7970732.
- ↑ "Entrez Gene: FUS fusion (involved in t(12;16) in malignant liposarcoma)".
- 1 2 3 4 Crozat A, Aman P, Mandahl N, Ron D (June 1993). "Fusion of CHOP to a novel RNA-binding protein in human myxoid liposarcoma". Nature. 363 (6430): 640–4. doi:10.1038/363640a0. PMID 8510758.
- ↑ Mrózek K, Karakousis CP, Bloomfield CD (April 1993). "Chromosome 12 breakpoints are cytogenetically different in benign and malignant lipogenic tumors: localization of breakpoints in lipoma to 12q15 and in myxoid liposarcoma to 12q13.3". Cancer Res. 53 (7): 1670–5. PMID 8453640.
- ↑ Aman P, Panagopoulos I, Lassen C, Fioretos T, Mencinger M, Toresson H, Höglund M, Forster A, Rabbitts TH, Ron D, Mandahl N, Mitelman F (October 1996). "Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS". Genomics. 37 (1): 1–8. doi:10.1006/geno.1996.0513. PMID 8921363.
- 1 2 Morohoshi F, Ootsuka Y, Arai K, Ichikawa H, Mitani S, Munakata N, Ohki M (October 1998). "Genomic structure of the human RBP56/hTAFII68 and FUS/TLS genes". Gene. 221 (2): 191–8. doi:10.1016/S0378-1119(98)00463-6. PMID 9795213.
- 1 2 Bertolotti A, Bell B, Tora L (December 1999). "The N-terminal domain of human TAFII68 displays transactivation and oncogenic properties". Oncogene. 18 (56): 8000–10. doi:10.1038/sj.onc.1203207. PMID 10637511.
- ↑ Rappsilber J, Friesen WJ, Paushkin S, Dreyfuss G, Mann M (July 2003). "Detection of arginine dimethylated peptides by parallel precursor ion scanning mass spectrometry in positive ion mode". Anal. Chem. 75 (13): 3107–14. doi:10.1021/ac026283q. PMID 12964758.
- ↑ Iko Y, Kodama TS, Kasai N, Oyama T, Morita EH, Muto T, Okumura M, Fujii R, Takumi T, Tate S, Morikawa K (October 2004). "Domain architectures and characterization of an RNA-binding protein, TLS". J. Biol. Chem. 279 (43): 44834–40. doi:10.1074/jbc.M408552200. PMID 15299008.
- ↑ Zinszner H, Albalat R, Ron D (November 1994). "A novel effector domain from the RNA-binding protein TLS or EWS is required for oncogenic transformation by CHOP". Genes Dev. 8 (21): 2513–26. doi:10.1101/gad.8.21.2513. PMID 7958914.
- ↑ Calvio C, Neubauer G, Mann M, Lamond AI (September 1995). "Identification of hnRNP P2 as TLS/FUS using electrospray mass spectrometry". RNA. 1 (7): 724–33. PMC 1369314
 . PMID 7585257.
. PMID 7585257. - ↑ Zinszner H, Sok J, Immanuel D, Yin Y, Ron D (August 1997). "TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling". J. Cell. Sci. 110 (15): 1741–50. PMID 9264461.
- ↑ Perrotti D, Bonatti S, Trotta R, Martinez R, Skorski T, Salomoni P, Grassilli E, Lozzo RV, Cooper DR, Calabretta B (August 1998). "TLS/FUS, a pro-oncogene involved in multiple chromosomal translocations, is a novel regulator of BCR/ABL-mediated leukemogenesis". EMBO J. 17 (15): 4442–55. doi:10.1093/emboj/17.15.4442. PMC 1170776
 . PMID 9687511.
. PMID 9687511. - ↑ Baechtold H, Kuroda M, Sok J, Ron D, Lopez BS, Akhmedov AT (November 1999). "Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLS/FUS and is able to promote D-loop formation". J. Biol. Chem. 274 (48): 34337–42. doi:10.1074/jbc.274.48.34337. PMID 10567410.
- ↑ Wang X, Arai S, Song X, Reichart D, Du K, Pascual G, Tempst P, Rosenfeld MG, Glass CK, Kurokawa R (July 2008). "Induced ncRNAs allosterically modify RNA-binding proteins in cis to inhibit transcription". Nature. 454 (7200): 126–30. doi:10.1038/nature06992. PMC 2823488
 . PMID 18509338.
. PMID 18509338. - ↑ Lerga A, Hallier M, Delva L, Orvain C, Gallais I, Marie J, Moreau-Gachelin F (March 2001). "Identification of an RNA binding specificity for the potential splicing factor TLS". J. Biol. Chem. 276 (9): 6807–16. doi:10.1074/jbc.M008304200. PMID 11098054.
- ↑ Fujii R, Takumi T (December 2005). "TLS facilitates transport of mRNA encoding an actin-stabilizing protein to dendritic spines". J. Cell. Sci. 118 (Pt 24): 5755–65. doi:10.1242/jcs.02692. PMID 16317045.
- ↑ Takahama K, Kino K, Arai S, Kurokawa R, Oyoshi T (2008). "Identification of RNA binding specificity for the TET-family proteins". Nucleic Acids Symp Ser (Oxf) (52): 213–4. doi:10.1093/nass/nrn108. PMID 18776329.
- ↑ Law WJ, Cann KL, Hicks GG (March 2006). "TLS, EWS and TAF15: a model for transcriptional integration of gene expression". Brief Funct Genomic Proteomic. 5 (1): 8–14. doi:10.1093/bfgp/ell015. PMID 16769671.
- ↑ Powers CA, Mathur M, Raaka BM, Ron D, Samuels HH (January 1998). "TLS (translocated-in-liposarcoma) is a high-affinity interactor for steroid, thyroid hormone, and retinoid receptors". Mol. Endocrinol. 12 (1): 4–18. doi:10.1210/me.12.1.4. PMID 9440806.
- 1 2 Hallier M, Lerga A, Barnache S, Tavitian A, Moreau-Gachelin F (February 1998). "The transcription factor Spi-1/PU.1 interacts with the potential splicing factor TLS". J. Biol. Chem. 273 (9): 4838–42. doi:10.1074/jbc.273.9.4838. PMID 9478924.
- 1 2 Uranishi H, Tetsuka T, Yamashita M, Asamitsu K, Shimizu M, Itoh M, Okamoto T (April 2001). "Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-kappa B p65-mediated transcription as a coactivator". J. Biol. Chem. 276 (16): 13395–401. doi:10.1074/jbc.M011176200. PMID 11278855.
- ↑ Bertolotti A, Lutz Y, Heard DJ, Chambon P, Tora L (September 1996). "hTAF(II)68, a novel RNA/ssDNA-binding protein with homology to the pro-oncoproteins TLS/FUS and EWS is associated with both TFIID and RNA polymerase II". EMBO J. 15 (18): 5022–31. PMC 452240
 . PMID 8890175.
. PMID 8890175. - ↑ Bertolotti A, Melot T, Acker J, Vigneron M, Delattre O, Tora L (March 1998). "EWS, but not EWS-FLI-1, is associated with both TFIID and RNA polymerase II: interactions between two members of the TET family, EWS and hTAFII68, and subunits of TFIID and RNA polymerase II complexes". Mol. Cell. Biol. 18 (3): 1489–97. PMC 108863
 . PMID 9488465.
. PMID 9488465. - 1 2 Yang L, Embree LJ, Hickstein DD (May 2000). "TLS-ERG leukemia fusion protein inhibits RNA splicing mediated by serine-arginine proteins". Mol. Cell. Biol. 20 (10): 3345–54. doi:10.1128/MCB.20.10.3345-3354.2000. PMC 85627
 . PMID 10779324.
. PMID 10779324. - ↑ Tan AY, Manley JL (January 2010). "TLS inhibits RNA polymerase III transcription". Mol. Cell. Biol. 30 (1): 186–96. doi:10.1128/MCB.00884-09. PMC 2798296
 . PMID 19841068.
. PMID 19841068. - ↑ Kwiatkowski TJ, Bosco DA, Leclerc AL, Tamrazian E, Vanderburg CR, Russ C, Davis A, Gilchrist J, Kasarskis EJ, Munsat T, Valdmanis P, Rouleau GA, Hosler BA, Cortelli P, de Jong PJ, Yoshinaga Y, Haines JL, Pericak-Vance MA, Yan J, Ticozzi N, Siddique T, McKenna-Yasek D, Sapp PC, Horvitz HR, Landers JE, Brown RH (February 2009). "Mutations in the FUS/TLS Gene on Chromosome 16 Cause Familial Amyotrophic Lateral Sclerosis". Science. 323 (5918): 1205–1208. doi:10.1126/science.1166066. PMID 19251627.
- ↑ Vance C, Rogelj B, Hortobágyi T, De Vos KJ, Nishimura AL, Sreedharan J, Hu X, Smith B, Ruddy D, Wright P, Ganesalingam J, Williams KL, Tripathi V, Al-Saraj S, Al-Chalabi A, Leigh PN, Blair IP, Nicholson G, de Belleroche J, Gallo JM, Miller CC, Shaw CE (February 2009). "Mutations in FUS, an RNA processing protein, cause familial amyotrophic lateral sclerosis type 6". Science. 323 (5918): 1208–11. doi:10.1126/science.1165942. PMID 19251628.
- ↑ Mackenzie IR, Rademakers R, Neumann M (October 2010). "TDP-43 and FUS in amyotrophic lateral sclerosis and frontotemporal dementia". Lancet Neurol. 9 (10): 995–1007. doi:10.1016/S1474-4422(10)70195-2. PMID 20864052.
- ↑ Munoz DG, Neumann M, Kusaka H, Yokota O, Ishihara K, Terada S, Kuroda S, Mackenzie IR (November 2009). "FUS pathology in basophilic inclusion body disease". Acta Neuropathol. 118 (5): 617–27. doi:10.1007/s00401-009-0598-9. PMID 19830439.
- ↑ Neumann M, Rademakers R, Roeber S, Baker M, Kretzschmar HA, Mackenzie IR (November 2009). "A new subtype of frontotemporal lobar degeneration with FUS pathology". Brain. 132 (Pt 11): 2922–31. doi:10.1093/brain/awp214. PMC 2768659
 . PMID 19674978.
. PMID 19674978. - ↑ Neumann M, Roeber S, Kretzschmar HA, Rademakers R, Baker M, Mackenzie IR (November 2009). "Abundant FUS-immunoreactive pathology in neuronal intermediate filament inclusion disease". Acta Neuropathol. 118 (5): 605–16. doi:10.1007/s00401-009-0581-5. PMC 2864784
 . PMID 19669651.
. PMID 19669651. - ↑ Saunders LR, Perkins DJ, Balachandran S, Michaels R, Ford R, Mayeda A, Barber GN (August 2001). "Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR". J. Biol. Chem. 276 (34): 32300–12. doi:10.1074/jbc.M104207200. PMID 11438536.
- ↑ Wada K, Inoue K, Hagiwara M (August 2002). "Identification of methylated proteins by protein arginine N-methyltransferase 1, PRMT1, with a new expression cloning strategy". Biochim. Biophys. Acta. 1591 (1-3): 1–10. doi:10.1016/S0167-4889(02)00202-1. PMID 12183049.
- ↑ Lee J, Bedford MT (March 2002). "PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays". EMBO Rep. 3 (3): 268–73. doi:10.1093/embo-reports/kvf052. PMC 1084016
 . PMID 11850402.
. PMID 11850402. - ↑ Stelzl U, Worm U, Lalowski M, Haenig C, Brembeck FH, Goehler H, Stroedicke M, Zenkner M, Schoenherr A, Koeppen S, Timm J, Mintzlaff S, Abraham C, Bock N, Kietzmann S, Goedde A, Toksöz E, Droege A, Krobitsch S, Korn B, Birchmeier W, Lehrach H, Wanker EE (September 2005). "A human protein-protein interaction network: a resource for annotating the proteome". Cell. 122 (6): 957–68. doi:10.1016/j.cell.2005.08.029. PMID 16169070.
- ↑ Dormann D, Rodde R, Edbauer D, Bentmann E, Fischer I, Hruscha A, Than ME, Mackenzie IR, Capell A, Schmid B, Neumann M, Haass C (August 2010). "ALS-associated fused in sarcoma (FUS) mutations disrupt Transportin-mediated nuclear import". EMBO J. 29 (16): 2841–57. doi:10.1038/emboj.2010.143. PMC 2924641
 . PMID 20606625.
. PMID 20606625. - ↑ Brelstaff J, Lashley T, Holton JL, Lees AJ, Rossor MN, Bandopadhyay R, Revesz T (November 2011). "Transportin1: a marker of FTLD-FUS". Acta Neuropathol. 122 (5): 591–600. doi:10.1007/s00401-011-0863-6. PMID 21847626.
Further reading
- Pereira DS, Dorrell C, Ito CY, Gan OI, Murdoch B, Rao VN, Zou JP, Reddy ES, Dick JE (July 1998). "Retroviral transduction of TLS-ERG initiates a leukemogenic program in normal human hematopoietic cells". Proc. Natl. Acad. Sci. U.S.A. 95 (14): 8239–44. doi:10.1073/pnas.95.14.8239. PMC 20960
 . PMID 9653171.
. PMID 9653171. - Yi H, Fujimura Y, Ouchida M, Prasad DD, Rao VN, Reddy ES (March 1997). "Inhibition of apoptosis by normal and aberrant Fli-1 and erg proteins involved in human solid tumors and leukemias". Oncogene. 14 (11): 1259–68. doi:10.1038/sj.onc.1201099. PMID 9178886.
- Kaplowitz N, Ji C (2007). "Unfolding new mechanisms of alcoholic liver disease in the endoplasmic reticulum". J. Gastroenterol. Hepatol. 21 Suppl 3: S7–9. doi:10.1111/j.1440-1746.2006.04581.x. PMID 16958678.
- Panagopoulos I, Mandahl N, Ron D, Höglund M, Nilbert M, Mertens F, Mitelman F, Aman P (1995). "Characterization of the CHOP breakpoints and fusion transcripts in myxoid liposarcomas with the 12;16 translocation". Cancer Res. 54 (24): 6500–3. PMID 7987849.
- Ichikawa H, Shimizu K, Hayashi Y, Ohki M (1994). "An RNA-binding protein gene, TLS/FUS, is fused to ERG in human myeloid leukemia with t(16;21) chromosomal translocation". Cancer Res. 54 (11): 2865–8. PMID 8187069.
- Aman P, Panagopoulos I, Lassen C, Fioretos T, Mencinger M, Toresson H, Höglund M, Forster A, Rabbitts TH, Ron D, Mandahl N, Mitelman F (1997). "Expression patterns of the human sarcoma-associated genes FUS and EWS and the genomic structure of FUS". Genomics. 37 (1): 1–8. doi:10.1006/geno.1996.0513. PMID 8921363.
- Zinszner H, Sok J, Immanuel D, Yin Y, Ron D (1997). "TLS (FUS) binds RNA in vivo and engages in nucleo-cytoplasmic shuttling". J. Cell. Sci. 110 (15): 1741–50. PMID 9264461.
- Powers CA, Mathur M, Raaka BM, Ron D, Samuels HH (1998). "TLS (translocated-in-liposarcoma) is a high-affinity interactor for steroid, thyroid hormone, and retinoid receptors". Mol. Endocrinol. 12 (1): 4–18. doi:10.1210/me.12.1.4. PMID 9440806.
- Hallier M, Lerga A, Barnache S, Tavitian A, Moreau-Gachelin F (1998). "The transcription factor Spi-1/PU.1 interacts with the potential splicing factor TLS". J. Biol. Chem. 273 (9): 4838–42. doi:10.1074/jbc.273.9.4838. PMID 9478924.
- Zhang D, Paley AJ, Childs G (1998). "The transcriptional repressor ZFM1 interacts with and modulates the ability of EWS to activate transcription". J. Biol. Chem. 273 (29): 18086–91. doi:10.1074/jbc.273.29.18086. PMID 9660765.
- Yang L, Embree LJ, Tsai S, Hickstein DD (1998). "Oncoprotein TLS interacts with serine-arginine proteins involved in RNA splicing". J. Biol. Chem. 273 (43): 27761–4. doi:10.1074/jbc.273.43.27761. PMID 9774382.
- Bertrand P, Akhmedov AT, Delacote F, Durrbach A, Lopez BS (1999). "Human POMp75 is identified as the pro-oncoprotein TLS/FUS: both POMp75 and POMp100 DNA homologous pairing activities are associated to cell proliferation". Oncogene. 18 (31): 4515–21. doi:10.1038/sj.onc.1203048. PMID 10442642.
- Baechtold H, Kuroda M, Sok J, Ron D, Lopez BS, Akhmedov AT (1999). "Human 75-kDa DNA-pairing protein is identical to the pro-oncoprotein TLS/FUS and is able to promote D-loop formation". J. Biol. Chem. 274 (48): 34337–42. doi:10.1074/jbc.274.48.34337. PMID 10567410.
- Yang L, Embree LJ, Hickstein DD (2000). "TLS-ERG Leukemia Fusion Protein Inhibits RNA Splicing Mediated by Serine-Arginine Proteins". Mol. Cell. Biol. 20 (10): 3345–54. doi:10.1128/MCB.20.10.3345-3354.2000. PMC 85627
 . PMID 10779324.
. PMID 10779324. - Husi H, Ward MA, Choudhary JS, Blackstock WP, Grant SG (2000). "Proteomic analysis of NMDA receptor-adhesion protein signaling complexes". Nat. Neurosci. 3 (7): 661–9. doi:10.1038/76615. PMID 10862698.
- Uranishi H, Tetsuka T, Yamashita M, Asamitsu K, Shimizu M, Itoh M, Okamoto T (2001). "Involvement of the pro-oncoprotein TLS (translocated in liposarcoma) in nuclear factor-kappa B p65-mediated transcription as a coactivator". J. Biol. Chem. 276 (16): 13395–401. doi:10.1074/jbc.M011176200. PMID 11278855.
- Saunders LR, Perkins DJ, Balachandran S, Michaels R, Ford R, Mayeda A, Barber GN (2001). "Characterization of two evolutionarily conserved, alternatively spliced nuclear phosphoproteins, NFAR-1 and -2, that function in mRNA processing and interact with the double-stranded RNA-dependent protein kinase, PKR". J. Biol. Chem. 276 (34): 32300–12. doi:10.1074/jbc.M104207200. PMID 11438536.
- Lee J, Bedford MT (2002). "PABP1 identified as an arginine methyltransferase substrate using high-density protein arrays". EMBO Rep. 3 (3): 268–73. doi:10.1093/embo-reports/kvf052. PMC 1084016
 . PMID 11850402.
. PMID 11850402.