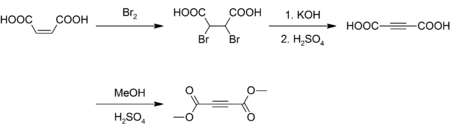Dimethyl acetylenedicarboxylate
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dimethyl but-2-ynedioate | |
| Other names
DMAD Acetylenedicarboxylic acid dimethyl ester | |
| Identifiers | |
| 762-42-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 12440 |
| ECHA InfoCard | 100.010.999 |
| RTECS number | ES0175000 |
| |
| |
| Properties | |
| C6H6O4 | |
| Molar mass | 142.11 g/mol |
| Appearance | Colorless liquid |
| Density | 1.1564 g/cm3 |
| Melting point | -18°C |
| Boiling point | 195 to 198 °C (383 to 388 °F; 468 to 471 K) (96–98° at 8 mm Hg) |
| Insoluble | |
| Solubility in other solvents | Soluble in most organic solvents |
| Refractive index (nD) |
1.447 |
| Structure | |
| 0 D | |
| Hazards | |
| Main hazards | Toxic gas |
| R-phrases | R34 |
| S-phrases | S23 S26 S27 S36/37/39 S45 |
| Flash point | 187 °C (369 °F; 460 K) |
| Related compounds | |
| Related compounds |
Methyl propiolate, Hexafluoro-2-butyne, Acetylene |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dimethyl acetylenedicarboxylate (DMAD) is an organic compound with the formula CH3O2CC2CO2CH3. It is a di-ester in which the ester groups are conjugated with a C-C triple bond. As such, the molecule is highly electrophilic, and is widely employed as a dienophile in cycloaddition reactions, such as the Diels-Alder reaction. It is also a potent Michael acceptor.[1][2] This compound exists as a colorless liquid at room temperature. This compound was used in the preparation of nedocromil.
Preparation
Although inexpensively available, DMAD is prepared today as it was originally. Maleic acid is brominated and the resulting dibromosuccinic acid is dehydrohalogenated with potassium hydroxide yielding acetylenedicarboxylic acid.[3][4] The acid is then esterified with methanol and sulfuric acid as a catalyst:[5]
Safety
DMAD is a lachrymator and a vesicant.
References
- ↑ Stelmach, J. E.; Winkler, J. D. "Dimethyl Acetylenedicarboxylate"in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ↑ Sahoo, Manoj (2007). "Dimethyl Acetylene Dicarboxylate". Synlett. 2007: 2142. doi:10.1055/s-2007-984894.
- ↑ Bandrowski, E. (1877). "Ueber Acetylendicarbonsäure". Berichte der deutschen chemischen Gesellschaft. 10: 838. doi:10.1002/cber.187701001231.
- ↑ Abbott, T. W.; Arnold, R. T.; Thompson, R. B. "Acetylenedicarboxylic acid". Org. Synth.; Coll. Vol., 2, p. 10
- ↑ Huntress, E. H. Lesslie, T. E.; Bornstein, J. "Dimethyl Acetylenedicarboxylate". Org. Synth.; Coll. Vol., 4, p. 329