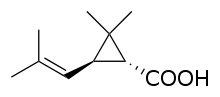
Chrysanthemic acid
 | |
| Names | |
|---|---|
| IUPAC name
2,2-Dimethyl-3-(2-methylprop-1-enyl)cyclopropane-1-carboxylic acid | |
| Identifiers | |
| 4638-92-0 (1R,3R) or (+)-trans | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1437285 |
| ChemSpider | 15876 (+)-trans 19543 (−)-cis |
| ECHA InfoCard | 100.022.788 |
| PubChem | 16747 (1R,3R) or (+)-trans 33607 (1S,3S) or (−)-trans 33606 (1R,3S) or (+)-cis 20755 (1S,3R) or (−)-cis |
| |
| |
| Properties | |
| C10H16O2 | |
| Molar mass | 168.24 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Chrysanthemic acid is an organic compound that is related to a variety of natural and synthetic insecticides. It is related to the pyrethrin I and II, as well as the pyrethroids. One of the four stereoisomers, (1R,3R)- or (+)-trans-chrysanthemic acid (pictured), is the acid part of the ester pyrethrin I, which occurs naturally in the seed cases of Chrysanthemum cinerariaefolium. Many synthetic pyrethroids, for example the allethrins, are esters of all four stereoisomers.
Biosynthesis
Chrysanthemic acid is derived from its pyrophosphate ester, which in turn is produced naturally from two molecules of dimethylallyl diphosphate.[1]

Industrial synthesis
Chrysanthemic acid is produced industrially in a cyclopropanation reaction of a diene as a mixture of cis- and trans isomers, followed by hydrolysis of the ester:[2]
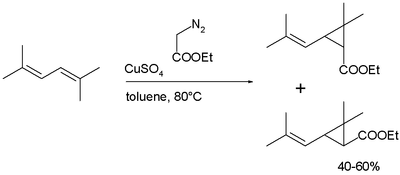
Many pyrethroids are accessible by re-esterification of chrysanthemic acid ethylester.
References
- ↑ Shattuck-Eidens DM, Wrobel WM, Peiser GD, Poulter CD (2001). "Chrysanthemyl diphosphate synthase: isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium". Proc. Natl. Acad. Sci. U.S.A. 98 (8): 4373–8. doi:10.1073/pnas.071543598. PMC 31842
 . PMID 11287653.
. PMID 11287653. - ↑ Kelly Lawrence F (1987). "A synthesis of chrysanthemic ester: An undergraduate experiment". J. Chem. Educ. 64 (12): 1061. doi:10.1021/ed064p1061.