Acid–base homeostasis
| Acids and bases |
|---|
| Acid types |
| Base types |
Acid–base homeostasis is the part of biologic homeostasis (See The extracellular fluid pH homeostat) concerning the proper balance between chemical acids and bases, also called body pH. The body is very sensitive to its extracellular pH level, so strong mechanisms exist to maintain it. Outside the acceptable range of pH, proteins are denatured and digested, enzymes lose their ability to function, and death may occur. The principles of general acid–base equilibrium apply in the physiology of living systems.
In various animals, including humans, the acid–base homeostasis system can be analyzed into 3 subsystems that are interrelated. They are the bicarbonate buffer system, the phosphate buffer system, and the protein buffer system. Respiratory compensation and renal compensation are two ways that the body can excrete excess molecules to maintain pH. Acidosis or alkalosis results if the balance is disturbed.
Acid–base balance
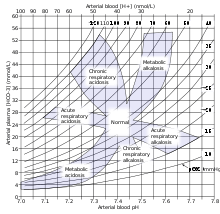
The body's acid–base balance is normally tightly regulated by buffering agents, the respiratory system, and the renal system, keeping the arterial blood pH between 7.36 and 7.42.[1][2] Several buffering agents that reversibly bind hydrogen ions and impede any change in pH exist. Extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphate act as intracellular buffers; the relationship between multiple buffers in the same solution is described by the isohydric principle. The bicarbonate buffering system is especially key, as carbon dioxide (CO2) can be shifted through carbonic acid (H
2CO
3) to hydrogen ions and bicarbonate (HCO−
3) as shown below.[3]
Acid–base imbalances that overcome the buffer system can be compensated in the short term by changing the rate of ventilation. This alters the concentration of carbon dioxide in the blood, shifting the above reaction according to Le Chatelier's principle, which in turn alters the pH. For instance, if the blood pH drops too low (acidemia), the body will compensate by increasing breathing[4] thereby expelling CO2, and shifting the above reaction to the left such that fewer hydrogen ions are free; thus the pH will rise back to normal. For alkalemia, the opposite occurs.
The kidneys are slower to compensate, but renal physiology has several powerful mechanisms to control pH by the excretion of excess acid or base. In response to acidosis, tubular cells reabsorb more bicarbonate from the tubular fluid, collecting duct cells secrete more hydrogen and generate more bicarbonate, and ammonia genesis leads to increased formation of the NH
3 buffer. In responses to alkalosis, the kidney may excrete more bicarbonate by decreasing hydrogen ion secretion from the tubular epithelial cells, and lowering rates of glutamine metabolism and ammonium excretion.
Imbalance
Acid–base imbalance occurs when a significant insult causes the blood pH to shift out of the normal range (7.35 to 7.45). In the fetus, the normal range differs based on which umbilical vessel is sampled (umbilical vein pH is normally 7.25 to 7.45; umbilical artery pH is normally 7.18 to 7.38).[5] An excess of acid in the blood is called acidemia and an excess of base is called alkalemia. The process that causes the imbalance is classified based on the etiology of the disturbance (respiratory or metabolic) and the direction of change in pH (acidosis or alkalosis). There are four basic processes: metabolic acidosis, respiratory acidosis, metabolic alkalosis, and respiratory alkalosis. One or a combination may occur at any given time.
References
- ↑ MedlinePlus Encyclopedia Blood gases
- ↑ Caroline, Nancy (2013). Nancy Caroline's Emergency care in the streets (7th ed.). Buffer systems: Jones & Bartlett Learning. pp. 347–349. ISBN 978-1449645861.
- ↑ Garrett, Reginald H.; Grisham, Charles M (2010). Biochemistry. Cengage Learning. p. 43. ISBN 978-0-495-10935-8.
- ↑ MedlinePlus Encyclopedia Metabolic acidosis
- ↑ Yeomans, ER; Hauth, JC; Gilstrap, LC III; Strickland DM (1985). "Umbilical cord pH, PCO2, and bicarbonate following uncomplicated term vaginal deliveries (146 infants)". Am J Obstet Gynecol. 151: 798–800. doi:10.1016/0002-9378(85)90523-x. PMID 3919587.
External links
- Stewart's original text at acidbase.org
- On-line text at AnaesthesiaMCQ.com
- Overview at kumc.edu
- Tutorial at acid-base.com
- Online acid–base physiology text
- Diagnoses at lakesidepress.com
- Interpretation at nda.ox.ac.uk