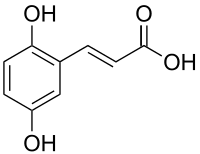
2,5-Dihydroxycinnamic acid
 | |
| Names | |
|---|---|
| IUPAC name
3-(2,5-dihydroxyphenyl)prop-2-enoic acid | |
| Other names
(2E)-3-(2,5-Dihydroxyphenyl)acrylic acid | |
| Identifiers | |
| 636-01-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 6442618 |
| PubChem | 181581 |
| |
| |
| Properties | |
| C9H8O4 | |
| Molar mass | 180.16 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
2,5-Dihydroxycinnamic acid is a hydroxycinnamic acid. It is an isomer of caffeic acid.
Preparation
2,5-Dihydroxycinnamic acid is produced by Elbs persulfate oxidation of o-Coumaric acid.[1][2]
See also
References
- ↑ Cain, J.C.; Greenaway, A.J. (1907). "Abstracts of Papers on Organic chemistry". Journal of the Chemical Society, abstracts. 92: A741–A812. doi:10.1039/CA9079200741.
- ↑ Otto, Neubauer; Flatow, L. (1907). "Synthesen von Alkaptonsäuren". Zeitschrift für Physiologische Chemie. 52: 375–398.
This article is issued from Wikipedia - version of the 2/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.