Zebularine
 | |
| Names | |
|---|---|
| IUPAC name
1-(β-D-Ribofuranosyl)-2(1H)-pyrimidinone | |
| Other names
Pyrimidin-2-one β-D-ribofuranoside | |
| Identifiers | |
| 3690-10-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 90372 |
| 4729 | |
| PubChem | 100016 |
| UNII | 7A9Y5SX0GY |
| |
| |
| Properties | |
| C9H12N2O5 | |
| Molar mass | 228.20 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Zebularine is a nucleoside analog of cytidine. It is a "transition state analog inhibitor of cytidine deaminase by binding to the active size as covalent hydrates. Also shown to inhibit DNA methylation and tumor growth both in vitro and in vivo."[1]
In a small study of mice with a defective Adenomatous polyposis coli gene, oral administration of zebularine to males had no effect on the overall methylation of DNA or the number of polyps, but in females the average number of polyps was reduced from 58 to 1.[2] It has therefore been suggested for drug use as a prototype of epigenetic therapy for cancer chemoprevention.[3]
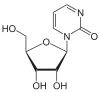

Zebularine (left) closely resembles cytidine (right), but lacks the 4'-amino group.
References
- ↑ "pyrimidin-2-one beta-ribofuranoside - Compound Summary". PubChem/National Center for Biotechnology Information.
- ↑ Christine B. Yoo; et al. (2008-09). "Long-term Epigenetic Therapy with Oral Zebularine Has Minimal Side Effects and Prevents Intestinal Tumors in Mice". Cancer Prev Res. 1: 233. doi:10.1158/1940-6207.capr-07-0008. Check date values in:
|date=(help) - ↑ Peter W. Laird (2005-04-15). "Cancer epigenetics". Hum. Mol. Genet. 14 (suppl 1): R65–R76. doi:10.1093/hmg/ddi113.
This article is issued from Wikipedia - version of the 9/21/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.