Xylenol
Xylenols are organic compounds with the formula (CH3)2C6H3OH. They are volatile colorless solids or oily liquids. They are derivatives of phenol with two methyl groups and a hydroxyl group. Six isomers exist, of which 2,6-xylenol with both methyl group in an ortho position with respect to the hydroxyl group is the most important. The name xylenol is a portmanteau of the words xylene and phenol.
2,4-dimethylphenol together with other xylenols and many other compounds are traditionally extracted from coal tar, the volatile materials obtained in the production of coke from coal. These residue contains a few percent by weight of xylenols as well as cresols and phenol. The main xylenols in such tar are the 3,5-, 2,4, and 2,3- isomers. 2,6-Xylenol is produced by methylation of phenol using methanol in the presence of metal oxide catalysts:[1]
- C6H5OH + 2 CH3OH → (CH3)2C6H3OH + 2 H2O
Properties
The physical properties of the six isomeric xylenols are similar.
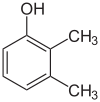
| Isomer | 2,6-Xylenol | 2,5-Xylenol | 2,4-Xylenol | 2,3-Xylenol | 3,4-Xylenol | 3,5-Xylenol |
| Structure |  |
 |
 |
 |
 |
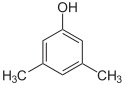 |
| CAS | 576-26-1 | 95-87-4 | 105-67-9 | 526-75-0 | 95-65-8 | 108-68-9 |
| Mp °C | 43-45 | 63 - 65 | 22-23 | 70-73 | 62 - 68 | 61-64 |
| Bp °C | 203 | 212 | 211-212 | 217 | 227 | 222 |
| pKa [2] | 10.59 | 10.22 | 10.45 | 10.50 | 10.32 | 10.15 |
| Density g/mL | 0.971 | 1.011 | ||||
Uses
Together with cresols and cresylic acid, xylenols are an important class of phenolics with great industrial importance. They are used in the manufacture of antioxidants. Xylenol orange is a redox indicator built on a xylenol skeleton. 2,6-Xylenol is a monomer for poly(p-phenylene oxide) (PEO) engineering resins through carbon-oxygen oxidative coupling.
References
- ↑ Helmut Fiegein "Cresols and Xylenols" in Ullmann's Encyclopedia of Industrial Chemistry, 2007; Wiley-VCH, Weinheim. doi:10.1002/14356007.a08_025
- ↑ CRC Handbook of Tables for Organic Compound Identification, Third Edition, 1984, ISBN 0-8493-0303-6.
See also
External links
- Datasheet 2,6 isomer
- Datasheet 2,5 isomer
- Datasheet 2,4 isomer
- Datasheet 2,3 isomer
- Datasheet 3,4 isomer
- Datasheet 3,5 isomer