Xanthotoxol
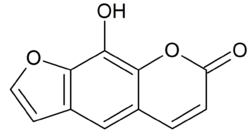 | |
| Names | |
|---|---|
| IUPAC name
9-hydroxyfuro[3,2-g]chromen-7-one | |
| Other names
8-Hydroxypsoralen 8-Hydroxypsoralene 8-Hydroxyfuranocoumarin | |
| Identifiers | |
| 2009-24-7 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 58600 |
| ECHA InfoCard | 100.016.295 |
| PubChem | 65090 |
| |
| |
| Properties | |
| C11H6O4 | |
| Molar mass | 202.16 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Xanthotoxol is a furanocoumarin. It is one of the major active ingredients in Cnidium monnieri.[1]
Metabolism
- Xanthotoxol O-methyltransferase (8-hydroxyfuranocoumarin 8-O-methyltransferase) is an enzyme that uses S-adenosyl methionine and xanthotoxol to produce S-adenosylhomocysteine and O-methylxanthotoxol (xanthotoxin or methoxsalen).
References
- ↑ Xanthotoxol exerts neuroprotective effects via suppression of the inflammatory response in a rat model of focal cerebral ischemia. He W, Chen W, Zhou Y, Tian Y and Liao F, Cell Mol Neurobiol., July 2013, volume 33, issue 5, pages 715-722, doi:10.1007/s10571-013-9939-2
This article is issued from Wikipedia - version of the 7/7/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.