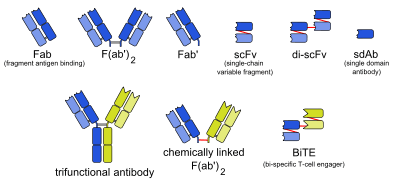
Trifunctional antibody

A trifunctional antibody is a monoclonal antibody with binding sites for two different antigens, typically CD3 and a tumor antigen, making it a type of bispecific monoclonal antibody. In addition, its intact Fc-part can bind to an Fc receptor on accessory cells like conventional monospecific antibodies. The net effect is that this type of drug links T cells (via CD3) and monocytes/macrophages, natural killer cells, dendritic cells or other Fc receptor expressing cells to the tumor cells, leading to their destruction.[1]
At an equivalent dose a trifunctional antibody is more potent (more than 1,000-fold) in eliminating tumor cells than conventional antibodies.[2] These drugs evoke the removal of tumor cells by means of (i) antibody-dependent cell-mediated cytoxicity, a process also described for conventional antibodies and more importantly by (ii) polyclonal cytotoxic T cell responses with emphasis on CD8 T cells. These trifunctional antibodies also elicit individual anti-tumor immune responses in cancer patients treated with e.g. catumaxomab; i.e. autologous antibodies as well as CD4 and CD8 T cells directed against the tumor were detected.[3][4] Furthermore, putative cancer stem cells from malignant ascites fluid were eliminated due to catumaxomab treatment.[5]
Catumaxomab, was the first to be approved for clinical use (in 2009 for the treatment of malignant ascites in cancer patients).
Examples include catumaxomab (EpCAM / CD3),[6][7] ertumaxomab (HER2/neu / CD3),[8] FBTA05 (CD20 / CD3, proposed trade name Lymphomun)[9][10] and TRBS07 (GD2 / CD3, proposed trade name Ektomab),[11] drugs against various types of cancer.
History
Trifunctional antibodies were the first type of bispecific monoclonal antibodies to be produced. The first concepts date back to the mid-1980s.[12][13] For over twenty years, no such antibody was approved for clinical use, mainly because of manufacturing difficulties. Immunogenicity results from the fact that appropriate parental antibodies are obtained from rat and mice. After application, the patient's immune system usually produces anti-drug antibodies, which represent early indicators for a beneficial clinical outcome.[14] Furthermore, despite the development of anti-drug antibody responses after the first catumaxomab application cycle a repeated cycle of catumaxomab also leads to a treatment success in recurrent malignant ascites.[15] Cross-linking leads to the release of cytokines, resulting in manageable adverse effects like fever, nausea and vomiting, that were generally reversible and mainly related to the immunological mode of action (e.g. catumaxomab).[16] Catumaxomab, which was approved in 2009 for the treatment of malignant ascites in cancer patients, satisfies these conditions. It was the first, and as of May 2011 the only approved one of these antibodies in clinical use.
Another way of immunotherapeutic intervention strategies is the exploration of bispecific antibodies with different structures, of which bi-specific T-cell engagers (BiTEs) have been produced since the mid-2000s.[17]
Production
At first, mouse hybridoma cells whose monoclonal antibodies target one of the desired antigens are produced. Independently, rat hybridoma cells targeting the other antigen are produced. These two cell types are hybridised, yielding hybrid-hybridomas or quadromas, which produce hybrid (trifunctional) antibody as well as pure mouse and pure rat antibody. The trifunctional antibody is extracted chromatographically with protein A.

Using two different species (mouse and rat) has the advantage that less mismatched antibodies are produced because rat light chains preferably pair with rat heavy chains, and mouse light chains with mouse heavy chains. Single species (mouse/mouse or rat/rat) quadromas, by contrast, produce up to ten different kinds of antibody, most of which have mismatched heavy or light chains, or both.[18]
References
- ↑ Chames, P; Baty, D (2009). "Bispecific antibodies for cancer therapy: the light at the end of the tunnel". MAbs. 1 (6): 1–9. doi:10.4161/mabs.1.6.10015. PMC 2791310
 . PMID 20073127.
. PMID 20073127. - ↑ Jaeger, M; Ruf, P; Hess, J; Lindhofer, H; et al. (2009). "The trifunctional antibody ertumaxomab destroys tumor cells that express low levels of human epidermal growth factor receptor". Cancer Research. 69 (10): 4270–4276. doi:10.1158/0008-5472.CAN-08-2861. PMID 19435924.
|first2=missing|last2=in Authors list (help) - ↑ Reinhard, H; et al. (2011). "The effect of trifunctional anti-EpCAM antibody catumaxomab on the development of tumor-specific immune responses in patients with gastric cancer". Journal of Clinical Oncology. 29 (suppl abstr 2601).
- ↑ Ruf, P; et al. (2011). "Humoral tumor-associated immune responses induced by catumaxomab in patients with malignant ascites". Journal of Clinical Oncology. 29 (suppl abstr 2575).
- ↑ Lindhofer, H; et al. (2009). "Elimination of cancer stem cells (CD133+/EpCAM+) from malignant ascites by the trifunctional antibody catumaxomab: results from a pivotal phase II/III study". Journal of Clinical Oncology. 27 (15s suppl abstr 3014).
- ↑ Shen, J; Zhu, Z (2008). "Catumaxomab, a rat/murine hybrid trifunctional bispecific monoclonal antibody for the treatment of cancer". Current Opinion in Molecular Therapeutics. 10 (3): 273–284. PMID 18535935.
- ↑ Sebastian, M; Friccius-Quecke, H; Jäger, M; Lindhofer, H; Kanniess, F; Wiewrodt, R; Thiel, E; et al. (2007). "Treatment of non-small cell lung cancer patients with the trifunctional monoclonal antibody catumaxomab (anti-EpCAM x anti-CD3): a phase I study". Cancer Immunology Immunotherapy. 56 (10): 1637–1644. doi:10.1007/s00262-007-0310-7. PMID 17410361.
- ↑ Kiewe, P; Kahlert, S; Heinrigs, M; Rack, B; Marmé, A; Korfel, A; Jäger, M; et al. (2006). "Phase I trial of the trifunctional antibody anti-HER2/neu x anti-CD3 antibody ertumaxomab in metastatic breast cancer". Clinical Cancer Research. 12 (10): 3085–3091. doi:10.1158/1078-0432.CCR-05-2436. PMID 16707606.
- ↑ Buhmann, R; Stanglmaier, M; Yang, T; Faltin, M; Bund, D; Lindhofer, H; Kolb, HJ; et al. (2008). "Immunotherapy of recurrent B–cell malignancies after allo SCT with Bi20 (FBTA05), a trifunctional anti-CD3 x anti-CD20 antibody and donor lymphocyte infusion". Bone Marrow Transplantation. 43 (5): 383–397. doi:10.1038/bmt.2008.323. PMID 18850012.
|first2=missing|last2=in Authors list (help) - ↑ Boehrer, S; Mueller, Tina; Atz, Judith; Chow, Kai Uwe; et al. (2011). "Cytotoxic effects of the trifunctional bispecific antibody FBTA05 in ex-vivo cells of chronic lymphocytic leukaemia depend on immune-mediated mechanisms". Anti-Cancer Drugs. 12 (10): 3085–3091. doi:10.1097/CAD.0b013e328344887f.
|first2=missing|last2=in Authors list (help) - ↑ Ruf, P; Ellwart, J; Wosch, S; Kusterer, E; Lindhofer, H; et al. (2004). "Two new trifunctional antibodies for the therapy of human malignant melanoma". International Journal of Cancer. 108 (5): 725–732. doi:10.1002/ijc.11630. PMID 14696099.
|first2=missing|last2=in Authors list (help) - ↑ Staerz, UD; Kanagawa, O; Bevan, MJ (1985). "Hybrid antibodies can target sites for attack by T cells". Nature. 314 (6012): 628–31. Bibcode:1985Natur.314..628S. doi:10.1038/314628a0. PMID 2859527.
- ↑ Mueller, D; Kontermann, RE (2010). "Bispecific antibodies for cancer immunotherapy". BioDrugs. 24 (2): 89–98. doi:10.2165/11530960-000000000-00000. PMID 20199124.
- ↑ Ott; et al. (2010). "The trifunctional antibody catumaxomab: correlation between immunological response and clinical outcome – new analysis of pivotal phase II/III study". Journal of Clinical Oncology. 28 (15s suppl abstr 2551).
- ↑ Pietzner, K; Schoberth, A; Oskay-Özcelik, G; Kuhberg, M; Lindhofer, H; Sehouli, J; et al. (2011). "First patient treated with re-challenge of catumaxomab in recurrent malignant ascites: a case report". Medical Oncology. 29 (2): 1391. doi:10.1007/s12032-011-9961-5. PMID 21544631.
|first2=missing|last2=in Authors list (help) - ↑ Heiss, MM; Koralewski, P; Kutarska, E; Kolesnik, OO; Ivanchenko, VV; Dudnichenko, AS; Aleknaviciene, B; et al. (2010). "The trifunctional antibody catumaxomab for the treatment of malignant ascites due to the epithelial cancer: results of a prospective randomized phase II/III trial". International Journal of Cancer. 127 (9): 2209–2221. doi:10.1002/ijc.25423. PMC 2958458
 . PMID 20473913.
. PMID 20473913. - ↑ Kufer, P; Lutterbüse, R; Baeuerle, PA (2004). "A revival of bispecific antibodies". Trends in Biotechnology. 22 (5): 238–44. doi:10.1016/j.tibtech.2004.03.006. PMID 15109810.
- ↑ Lindhofer, H; Mocikat, R; Steipe, B; Thierfelder, S (1995). "Preferential species-restricted heavy/light chain pairing in rat/mouse quadromas. Implications for a single-step purification of bispecific antibodies". Journal of immunology (Baltimore, Md. : 1950). 155 (1): 219–25. PMID 7602098.
Further reading
- Choi, BD; et, al.; Bigner, DD; Mehta, AI; Kuan, CT; Sampson, JH (2011). "Bispecific antibodies engage T cells for antitumor immunotherapy". Expert Opin Biol Ther. 11 (7): 1–11. doi:10.1517/14712598.2011.572874. PMID 21449821.
- Thakur, A; Lum, LG (2010). "Cancer therapy with bispecific antibodies: Clinical experience". Current Opinion in Molecular Therapeutics. 12 (3): 340–349. PMID 20521223.