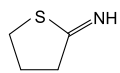
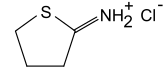
2-Iminothiolane
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
Dihydro-2(3H)-thiophenimine hydrochloride | |||
Other names
| |||
| Identifiers | |||
| 4781-83-3 (Hydrochloride) | |||
| ECHA InfoCard | 100.226.745 | ||
| PubChem | 13166855 | ||
| Properties | |||
| C4H7NS·HCl | |||
| Molar mass | 137.63 (Hydrochloride)[1] 101.17 (Free base) | ||
| Appearance | Powder | ||
| Boiling point | 198–201[1] °C (388–394 °F; 471–474 K) (Hydrochloride) | ||
| 100 mg/mL[1] | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
2-Iminothiolane is a cyclic thioimidate compound also known as Traut's reagent. It is a thiolating reagent that reacts with primary amine groups, such as those of amino acids, to form sulfhydryl groups.
Application
2-Iminothiolane reacts witih primary amines efficienty at pH 7 to 9, creating aminidine compounds with a sulfhydryl group. Thus it allows for crosslinking or labeling of molecules such as proteins through use of disulfide or thioether conjugation. It was first used to thiolate a subunit of ribosome in E. coli in 1973 by Robert Traut, its namesake, and his colleagues.[2]
It also reacts with aliphatic and phenolic hydroxyl groups at high pH, albeit at a much slower rate.[3]

The reaction of 2-iminothiolane with an amine group of a peptide.
References
- 1 2 3 Sigma-Aldrich Co., product no. I6256. Retrieved on July 06, 2015.
- ↑ R. R. Traut et al., Methyl 4-mercaptobutyrimidate as a cleavable cross-linking reagent and its application to the Escherichia coli 30S ribosome, Biochemistry 12, 1973, pp. 3266–3273, PMID 4581787.
- ↑ "Traut's Reagent Instructions" (PDF). Thermo Scientific. Retrieved 2015-07-08.
This article is issued from Wikipedia - version of the 6/20/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.