Thiuram disulfide

General structure of a thiuram disulfide
Thiuram disulfides are a class of organic disulfides that have the general structural formula shown to the right.
Preparation
They are prepared from the oxidation of their corresponding sodium salts of thiocarbamates with iodine:
- 2 NaS2CNR2 + I2 → R2NC(S)S-SC(S)NR2 + 2 NaI (where R is an alkyl group)
Example
The tetraethylthiuram disulfide compound, known as Disulfiram, is commonly used to treat chronic alcoholism by producing an acute sensitivity to alcohol ingestion by blocking acetaldehyde dehydrogenase conversion of acetaldehyde leading to a higher concentration of the aldehyde in the blood producing symptoms of a severe hangover.
Allergy
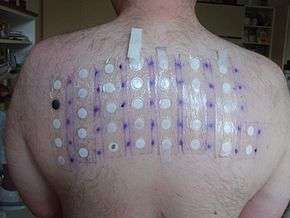
[Patch test]
In 2005–06, it was the 13th-most-prevalent allergen in patch tests (3.9%).[1]
References
- ↑ Zug KA, Warshaw EM, Fowler JF Jr, Maibach HI, Belsito DL, Pratt MD, Sasseville D, Storrs FJ, Taylor JS, Mathias CG, Deleo VA, Rietschel RL, Marks J. Patch-test results of the North American Contact Dermatitis Group 2005–2006. Dermatitis. 2009 May–Jun;20(3):149-60.
This article is issued from Wikipedia - version of the 5/5/2014. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.