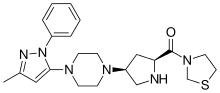
Teneligliptin
 | |
| Clinical data | |
|---|---|
| Trade names | Tenelia |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 760937-92-6 |
| PubChem (CID) | 11949652 |
| ChemSpider | 10123963 |
| Chemical and physical data | |
| Formula | C22H30N6OS |
| Molar mass | 426.58 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
Teneligliptin (INN; trade name Tenelia) is a pharmaceutical drug for the treatment of type 2 diabetes mellitus. It belongs to the class of anti-diabetic drugs known as dipeptidyl peptidase-4 inhibitors or "gliptins".[1]
Creation
It was created by Mitsubishi Tanabe Pharma and launched in September 2012 by both Mitsubishi Tanabe Pharma and Daiichi Sankyo in Japan.[2]
Licensing and use
Japan/Korea
It is approved for use in Japan, Korea and India. [3]
Pharmacology
Teneligliptin has unique J shaped or anchor locked domain structure because of which it has a potent inhibition of DPP 4 enzyme.
Teneligliptin significantly controls glycemic parameters with safety. No dose adjustment is required in renally impaired patients.[4]
References
- ↑ Kishimoto, M (2013). "Teneligliptin: A DPP-4 inhibitor for the treatment of type 2 diabetes". Diabetes, metabolic syndrome and obesity : targets and therapy. 6: 187–95. doi:10.2147/DMSO.S35682. PMC 3650886
 . PMID 23671395.
. PMID 23671395. - ↑ http://www.daiichisankyo.com/media_investors/media_relations/press_releases/detail/006054.html
- ↑ Joanne Bronson; Amelia Black; T. G. Murali Dhar; Bruce A. Ellsworth; J. Robert Merritt. "Teneligliptin (Antidiabetic), Chapter: To Market, To Market - 2012". Annual Reports in Medicinal Chemistry. 48: 523–524. doi:10.1016/b978-0-12-417150-3.00028-4.
- ↑ Biochemical and Biophysical Research Communications, 2013, 434(2), 191-196
This article is issued from Wikipedia - version of the 11/7/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.