TAPS (buffer)
 | |
| Names | |
|---|---|
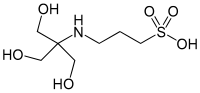
| IUPAC name
3-[[1,3-dihydroxy-2-(hydroxymethyl)propan-2-yl]amino]propane-1-sulfonic acid | |
| Other names
N-Tris(hydroxymethyl)methyl-3-aminopropanesulfonic acid | |
| Identifiers | |
| 29915-38-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 108495 |
| ECHA InfoCard | 100.045.398 |
| PubChem | 121591 |
| |
| |
| Properties | |
| C7H17NO6S | |
| Molar mass | 243.28 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
TAPS is used to make buffer solutions. It has a pKa value of 8.44 (ionic strength I=0, 25 °C).[1] It can be used to make buffer solutions in the pH range 7.7–9.1.
The pH (and pKa at I≠0) of the buffer solution changes with concentration and temperature, and this effect may be predicted using online calculators. [2] Binds Co(II), Ni(II). [3]
References
- ↑ Goldberg, R.; Kishore, N.; Lennen, R. (2002). "Thermodynamic Quantities for the Ionization Reactions of Buffers" (PDF). J. Phys. Chem. Ref. Data. 31: 231–370. doi:10.1063/1.1416902.
- ↑ "Biological buffers". REACH Devices.
- ↑ Machado, C. M. M.; Gameiro, P.; Soares, H. M. (2008). J. Solution Chem. 37 (5): 603–617. Missing or empty
|title=(help)
This article is issued from Wikipedia - version of the 6/18/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.