Synthetic musk

Synthetic musks, known as white musks in the perfume industry, are a class of synthetic aromachemicals to emulate the scent of deer musk or other natural musk. Synthetic musks have a clean, smooth and sweet scent lacking the fecal/"animalic" notes of natural musks and are sometimes attributed as having notes of blackberry, ambrette or ambergris. These compounds are essential in modern perfumery and form the base note foundations of most perfume formulas. Most, if not all musk fragrance used in perfumery today is synthetic.
Synthetic musks can be divided into three major classes — aromatic nitro musks, polycyclic musk compounds, and macrocyclic musk compounds.[1] The first two groups have broad uses in industry ranging from cosmetics to detergents.
Nitro-musks
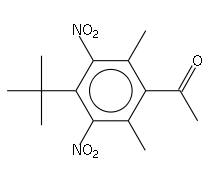
An artificial musk was obtained by Albert Baur in 1888 by condensing toluene with isobutyl bromide in the presence of aluminium chloride, and nitrating the product. It was discovered accidentally as a result of Baur's attempts at producing a more effective form of trinitrotoluene (TNT). It appears that the odour depends upon the symmetry of the three nitro groups.
- Moskene
Polycyclic musks
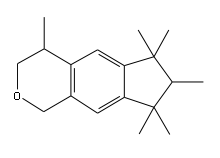
Polycyclic musks are defined by the International Fragrance Association (IFRA) as fragrance ingredients with the following olfactive and structural features:
- The predominant odour note is musk
- their structure shows some variations but has in common:
- A molecular weight of about 250 and a molecular formula of 17 or 18 carbons, 24 to 26 hydrogens and one oxygen
- A bi-cyclic aromatic structure of the indane or tetraline type, which is substituted with a combination of an acetyl group or a pyran ring in combination with methyl, isopropyl and/or t-butyl groups.
Polycylic musks that fit this definition are:
- Galaxolide (HHCB)
- Tonalide (Musk Plus, AHTN)
- Phantolide (AHMI)
- Celestolide (Crysolide, ADBI)
- Traseolide (ATII)
- Cashmeran
The creation of this class of musks was largely prompted through the need for eliminating the nitro functional group from nitro-musks due to their photochemical reactivity and their instability in alkaline media. This was shown to be possible through the discovery of ambral, a non-nitro aromatic musk, which promoted research in the development of nitro-free musks. This led to the eventual discovery of phantolide, so named due to its commercialization by Givaudan without initial knowledge of its chemical structure (elucidated 4 years later). While poorer in smell strength, the performance and stability of this compound class in harsh detergents led to its common use, which spurred further development of other polycyclic musks including Galaxolide.[2]
Macrocyclic musks
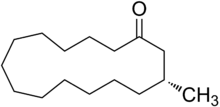
A class of artificial musk consisting of a single ring composed of more than 6 carbons (often 10-15). Of all artificial musks, these most resemble the primary odoriferous compound from Tonkin musk in its "large ringed" structure. While the macrocyclic musks extracted from plants consists of large ringed lactones, all animal derived macrocyclic musks are ketones.[2]
Although muscone, the primary macrocyclic compound of musk was long known, it was only in 1926 that Leopold Ruzicka was able to synthesize this compound in very small quantities. Despite this discovery and the discovery of other pathways for synthesis of macrocyclic musks, compound of this class were not commercially produced and commonly utilized until the late 1990s due to difficulties in their synthesis and consequently higher price.[3]
About half the human population are anosmic (unable to smell) to macrocyclic musks, possibly due to its high molecular weight. Common macrocyclic musks include:
- Ethylene brassilate
- Globalide (Habanolide)
- Ambrettolide
- Muscone
- Thibetolide (Exaltolide)
- Velvione
Alicyclic musks
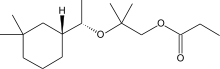
Alicyclic musks, otherwise known as cycloalkyl ester or linear musks, are a relatively novel class of musk compounds. The first compound of this class was introduced 1975 with Cyclomusk, though similar structures were noted earlier in citronellyl oxalate and Rosamusk.[4] Alicyclic musks are dramatically different in structure than previous musks (aromatic, polycyclic, macrocyclic) in that they are modified alkyl esters.[5] Although they were discovered prior to 1980, it was only in 1990 with the discovery and introduction of Helvetolide at Firmenich that a compound of this class was produced at a commercial scale.[4] Romandolide, a more ambrette and less fruity alicyclic musk compared to Helvetolide, was introduced ten years later.[5]
Environmental and health issues
Significant research on polycyclic musks and nitromusks has taken place since the 1990s in biomonitoring studies,.[1][6] They have also been detected in wastewater treatment plant sludges as a result of their removal in sewage treatment plants from domestic and industrial wastewater.[7]
All of the studies on polycylic musks, which only found low levels of polycyclic musks, have been incorporated in various safety reviews by authorities such as the European Scientific Committee on Cosmetics and Non-Food Products (SCCNFP; renamed recently as the Scientific Committee on Consumer Safety, or SCCS) and the US EPA’s Toxic Substance Control Act Work Plan Chemicals Program.
The outcome of those official reviews for the major polycyclic musks, Galaxolide (HHCB) and Tonalide (AHTN), is that these materials are safe for use in consumer products,[8][9][10][11][12][13][14][15] and for the environment,[12][13][16][17][18] without any need for risk reduction measures. Both Galaxolide (HHCB) and Tonalide (AHTN) have been registered under the EU REACH program[19] (CAS 1222-05-5 and 21145-77-7 respectively).
See also
References
- 1 2 Rimkus, Gerhard G. (Ed.); Cornelia Sommer (2004). "The Role of Musk and Musk Compounds in the Fragrance Industry". Synthetic Musk Fragrances in the Environment (Handbook of Environmental Chemistry). Springer. ISBN 3-540-43706-1.
- 1 2 Rowe, David J. (Ed.); Philip Kraft (2004). "Chapter 7. Aroma Chemicals IV: Musks". Chemistry and Technology of Flavours and Fragrances. Blackwell. ISBN 0-8493-2372-X.
- ↑ Charles (Ed.), Sell; Charles Sell (2005). "Chapter 4. Ingredients for the Modern Perfumery Industry". The Chemistry of Fragrances (2nd ed.). Royal Society of Chemistry Publishing. ISBN 978-0-85404-824-3.
- 1 2 Kraft, Philip (2004). "'Brain Aided' Musk Design". Chemistry & Biodiversity. 1 (12): 1957–1974. doi:10.1002/cbdv.200490150. PMID 17191832.
- 1 2 Eh, Marcus (2004). "New Alicyclic Musks: The Fourth Generation of Musk Odorants". Chemistry & Biodiversity. 1 (12): 1975–1984. doi:10.1002/cbdv.200490151. PMID 17191833.
- ↑ Synthetic Musk Fragrances in Lake Michigan. Aaron M. Peck and Keri C. Hornbuckle , Environ. Sci. Technol., 2004, volume 38, issue 2, pages 367–372, doi:10.1021/es034769y
- ↑ A rapid method to measure the solid–water distribution coefficient (Kd) for pharmaceuticals and musk fragrances in sewage sludge. Thomas A. Ternes, Nadine Herrmann, Matthias Bonerz, Thomas Knacker, Hansruedi Siegrist and Adriano Joss, Water Research, November 2004, Volume 38, Issue 19, Pages 4075–4084, doi:10.1016/j.watres.2004.07.015
- ↑ http://www.epa.gov/oppt/existingchemicals/pubs/workplans.html
- ↑ http://ec.europa.eu/food/fs/sc/sccp/out176_en.pdf
- ↑ http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_086.pdf
- ↑ http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_094.pdf
- 1 2 http://echa.europa.eu/documents/10162/947def3b-bbbf-473b-bc19-3bda7a8da910
- 1 2 http://echa.europa.eu/documents/10162/26e223a9-eda9-4e79-8c4d-650d2a3c1124
- ↑ http://ec.europa.eu/health/archive/ph_risk/committees/04_scher/docs/scher_o_091.pdf
- ↑ http://ec.europa.eu/health/archive/ph_risk/committees/04_scher/docs/scher_o_061.pdf
- ↑ http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_096.pdf
- ↑ http://ec.europa.eu/health/ph_risk/committees/04_scher/docs/scher_o_097.pdf
- ↑ http://www.epa.gov/oppt/existingchemicals/pubs/HHCB%20WP%20RA%20FINAL%2008_27_14.pdf
- ↑ http://echa.europa.eu/information-on-chemicals/registered-substances