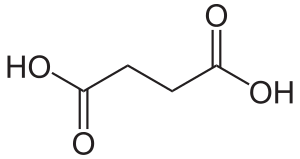
Succinic acid
 | |
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Butanedioic acid[1] | |
| Other names
Succinic acid[1] Ethane-1,2-dicarboxylic acid | |
| Identifiers | |
| 110-15-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:15741 |
| ChEMBL | ChEMBL576 |
| ChemSpider | 1078 |
| DrugBank | DB00139 |
| ECHA InfoCard | 100.003.402 |
| E number | E363 (antioxidants, ...) |
| 3637 | |
| PubChem | 1110 |
| UNII | AB6MNQ6J6L |
| |
| |
| Properties | |
| C4H6O4 | |
| Molar mass | 118.09 g·mol−1 |
| Density | 1.56 g/cm3[2] |
| Melting point | 184 °C (363 °F; 457 K)[2] |
| Boiling point | 235 °C (455 °F; 508 K)[2] |
| 58 g/L (20 °C)[2] or 100 mg/mL[3] | |
| Solubility in Methanol | 158 mg/mL[3] |
| Solubility in Ethanol | 54 mg/mL[3] |
| Solubility in Acetone | 27 mg/mL[3] |
| Solubility in Glycerol | 50 mg/mL[3] |
| Solubility in Ether | 8.8 mg/mL[3] |
| Acidity (pKa) | pKa1 = 4.2 pKa2 = 5.6 |
| Hazards | |
| Flash point | 206 °C (403 °F; 479 K)[2] |
| Related compounds | |
| Other anions |
sodium succinate |
| Related carboxylic acids |
propionic acid malonic acid butyric acid malic acid tartaric acid fumaric acid valeric acid glutaric acid |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Succinic acid (/səkˈsɪnᵻk/) is a dicarboxylic acid with chemical formula (CH2)2(CO2H)2. It is a white, odorless solid. In an aqueous solution, it ionizes to anions (that is, conjugates to a conjugate base) called succinate (/ˈsʌksᵻneɪt/), which plays a role in the citric acid cycle, an energy-yielding process in all living organisms. As a radical group it is called a succinyl (/ˈsʌksᵻnəl/) group. The name derives from Latin succinum, meaning amber, from which the acid may be obtained.
Production
Common industrial routes include partial hydrogenation of maleic acid, oxidation of 1,4-butanediol, and carbonylation of ethylene glycol.[4]
In a 2004 report, the United States Department of Energy identified bio-succinic acid as one of the renewable building block chemicals with the greatest technical feasibility and commercial potential.[5] Indeed, succinic acid is produced commercially through the fermentation of glucose from renewable feedstock.[6]
Historically known as spirit of amber,[7] succinic acid was originally obtained from amber by distillation.[7]
Reactions
Succinates
Salts formed by neutralizing succinic acid are called succinates. As a diprotic acid, succinic acid undergoes two successive reactions:
- (CH2)2(CO2H)2 → (CH2)2(CO2H)(CO2)− + H+
- (CH2)2(CO2H)(CO2)− → CH2)2(CO2)22− + H+
These anions, collectively called succinates, are also colorless, and can be isolated as the salts, e.g., Na(CH2)2(CO2H)(CO2) and Na2(CH2)2(CO2)22−.
Succinic acid also converts to diesters such are also referred to as succinates. One example is diethylsuccinate, (CH2CO2CH2CH3)2.
Other reactions
Succinic acid can be converted into fumaric acid by oxidation. The diethyl ester is a substrate in the Stobbe condensation. Dehydration of succinic acid gives succinic anhydride.[8]
Applications
Precursor to polymers, resins, and solvents
Succinic acid is a precursor to some polyesters. It is also a component of some alkyd resins.[4][9] Global 1,4-butanediol (BDO), derived from succinic acid, market size was estimated at USD 4.72 billion in 2013. BDO is a precursor to THF, a solvent and monomer. BDO is also a precursor to polybutylene terephthalate (PBT), an engineering-grade thermoplastic.[10] The automotive and electronics industries heavily rely on PBT to produce connectors, insulators, wheel covers, gearshift knobs and reinforcing beams.[11]
Food and dietary supplement
As a food additive and dietary supplement, succinic acid is generally recognized as safe for those uses by the U.S. Food and Drug Administration.[12] As an excipient in pharmaceutical products it is used to control acidity[13] and, more rarely, in effervescent tablets.[14]
Succinic acid is used in the food and beverage industry, primarily as an acidity regulator.[15] Global production is estimated at 16,000 to 30,000 tonnes a year, with an annual growth rate of 10%.[16]
Biochemistry
Succinate is an intermediate in the citric acid cycle. It serves as an electron donor to the electron transport chain:
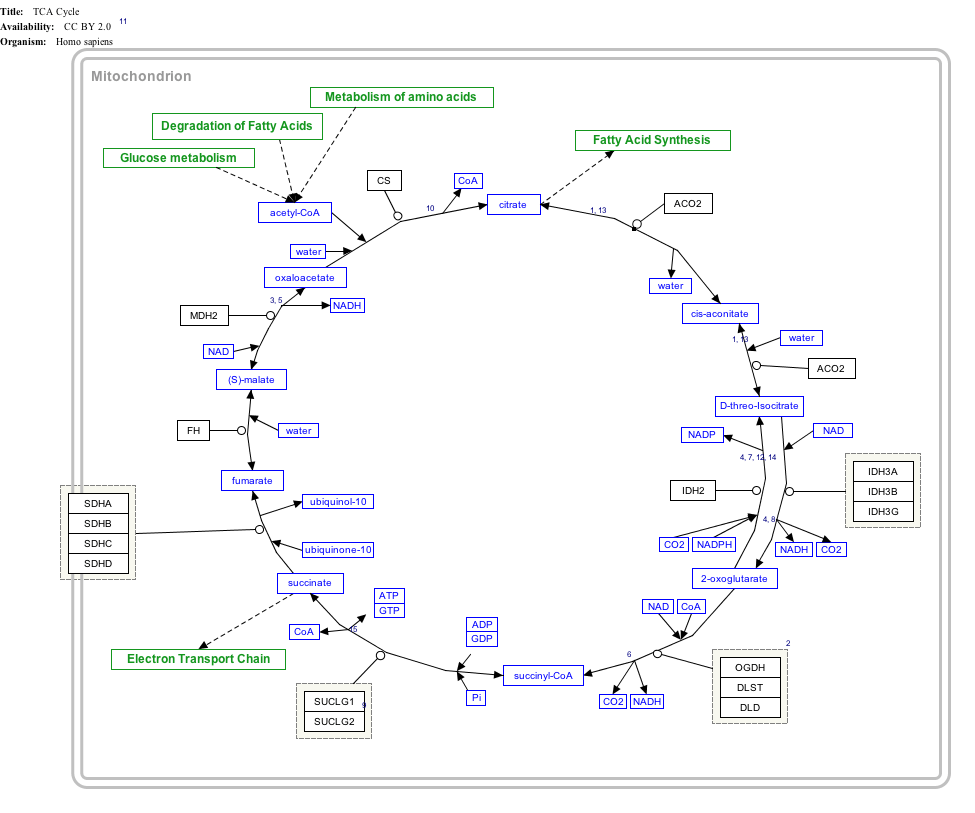
Click on genes, proteins and metabolites below to link to respective articles. [§ 1]
TCA Cycle edit
- ↑ The interactive pathway map can be edited at WikiPathways: "TCACycle_WP78".
This conversion is catalysed by the enzyme succinate dehydrogenase (or complex II of the mitochondrial ETC). The complex is a 4 subunit membrane-bound lipoprotein which couples the oxidation of succinate to the reduction of ubiquinone. Intermediate electron carriers are FAD and three 2Fe-2S clusters part of subunit B.
Fermentation
Succinic acid is created as a byproduct of the fermentation of sugar. It lends to fermented beverages such as wine and beer a common taste that is a combination of saltiness, bitterness and acidity.[17]
Safety
Succinic acid is an essential biosynthetic intermediate that occurs in all living creatures. Like most simple mono- and dicarboxylic acids, it is not dangerous but can be an irritant to skin and eyes.[18]
See also
- Oil of amber, procured by heating succinic acid.
References
- 1 2 Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 747. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- 1 2 3 4 5 Record in the GESTIS Substance Database of the IFA
- 1 2 3 4 5 6 "Product Information Sheet: Succinic Acid" (PDF). Sigma Aldrich. Retrieved 7 November 2015.
- 1 2 Boy Cornils, Peter Lappe (2005), "Dicarboxylic Acids, Aliphatic", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a08_523
- ↑ "Top Value Added Chemicals from Biomass, Volume 1: Results of Screening for Potential Candidates from Sugars and Synthesis Gas" (PDF). U.S. Department of Energy. November 1, 2004. Retrieved 2013-11-12.
- ↑ "Sustainability: Life Cycle Analysis - A Carbon Neutral Footprint". BioAmber Inc.
- 1 2 Chambers, E., ed. (1728). "Spirit of Amber". Cyclopaedia. p. 75.
- ↑ Louis F. Fieser, E. L. Martin, R. L. Shriner, and H. C. Struck (1932). "Succinic Anhydride". Org. Synth. 12: 66.; Coll. Vol., 2, p. 560.
- ↑ "BioAmber S-1/A". Securities and Exchange Commission. May 9, 2013. Retrieved 2013-11-11.
- ↑ "1,4-Butanediol (BDO)". Lyondell Basell. Retrieved 2013-11-12.
- ↑ "1,4-Butanediol (BDO) Market Analysis By Application (Tetrahydrofuran, Polybutylene Teraphthalate, Gamma-Butyrolactone & Polyurethanes), And Segment Forecasts To 2020". Grand View Research. September 2015. Retrieved 2015-11-18.
- ↑ FDA GRAS Database. Succinic acid in the FDA SCOGS Database
- ↑ "Overview of pharmaceutical excipients used in tablets and capsules". Modern Medicine Network. 24 October 2008. Retrieved 7 November 2015.
- ↑ Lachman, Leon; Joseph B. Schwartz (1990). Pharmaceutical Dosage Forms--tablets. p. 288. ISBN 9780824780449.
- ↑ Zeikus, J. G.; Jain, M. K.; Elankovan, P. (1999). "Biotechnology of succinic acid production and markets for derived industrial products". Applied Microbiology and Biotechnology. 51 (5): 545. doi:10.1007/s002530051431.
- ↑ NNFCC Renewable Chemicals Factsheet: Succinic Acid
- ↑ Peynaud, Emile (1984). Knowing and Making Wine.
- ↑ "Safety Data Sheet Bio-Based Succinic Acid". BioAmber Inc. April 23, 2015. Retrieved 2015-12-03.
 This article incorporates text from a publication now in the public domain: Chambers, Ephraim, ed. (1728). "Amber". Cyclopædia, or an Universal Dictionary of Arts and Sciences. 1 (first ed.). James and John Knapton, et al. p. 75.
This article incorporates text from a publication now in the public domain: Chambers, Ephraim, ed. (1728). "Amber". Cyclopædia, or an Universal Dictionary of Arts and Sciences. 1 (first ed.). James and John Knapton, et al. p. 75.
External links
| Citric acid cycle metabolic pathway | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Oxaloacetate | Malate | Fumarate | Succinate | Succinyl-CoA | ||||||||||||
| |
|
|
|
|
||||||||||||
| |
|
|
|
|||||||||||||
| Acetyl-CoA | NADH + H+ | NAD+ | H2O | FADH2 | FAD | CoA + ATP(GTP) | Pi + ADP(GDP) | |||||||||
| + | H2O | |
|
NADH + H+ + CO2 | ||||||||||||
| CoA | NAD+ | |||||||||||||||
| |
H2O | |
H2O | |
NAD(P)+ | NAD(P)H + H+ | |
CO2 | |
|||||||
| |
|
|
|
|||||||||||||
| Citrate | cis-Aconitate | Isocitrate | Oxalosuccinate | α-Ketoglutarate | ||||||||||||