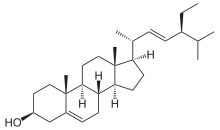
Stigmasterol
 | |
 | |
| Names | |
|---|---|
| IUPAC name
(3S,8S,9S,10R,13R,14S,17R)-17-[(E,2R,5S)-5-ethyl-6-methylhept-3-en-2-yl]-10,13-dimethyl-2,3,4,7,8,9,11,12,14,15,16,17-dodecahydro-1H-cyclopenta[a]phenanthren-3-ol | |
| Other names
Stigmasterin; Wulzen anti-stiffness factor | |
| Identifiers | |
| 83-48-7 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:28824 |
| ChEMBL | ChEMBL400247 |
| ChemSpider | 4444352 |
| ECHA InfoCard | 100.001.348 |
| PubChem | 5280794 |
| UNII | 99WUK5D0Y8 |
| |
| |
| Properties | |
| C29H48O | |
| Molar mass | 412.70 g·mol−1 |
| Appearance | White solid[1] |
| Melting point | 160 to 164 °C (320 to 327 °F; 433 to 437 K)[1] |
| Insoluble | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Stigmasterol (also known as Wulzen anti-stiffness factor) is a plant sterol, or phytosterol.
Discovery
Wulzen factor, as it was first known, was discovered by University of California physiologist Rosalind Wulzen (born 1886).[2]
Natural occurrences
Stigmasterol is an unsaturated phytosterol occurring in the plant fats or oils of soybean, calabar bean, and rape seed, and in a number of medicinal herbs, including the Chinese herbs Ophiopogon japonicus (Mai men dong), in Mirabilis jalapa[3] and American Ginseng.
Stigmasterol is also found in various vegetables, legumes, nuts, seeds, and unpasteurized milk. Pasteurization will inactivate stigmasterol. Edible oils contains higher amount than vegetables.[4] Phytosterols normally are broken down in the bile.
Uses
Stigmasterol is used as a precursor in the manufacture of semisynthetic progesterone,[5][6][7] a valuable human hormone that plays an important physiological role in the regulatory and tissue rebuilding mechanisms related to estrogen effects, as well as acting as an intermediate in the biosynthesis of androgens, estrogens, and corticoids. It is also used as the precursor of vitamin D3.[8]
The Upjohn company used stigmasterol as the starting raw material for the synthesis of cortisone.[9][10]
Research
Research has indicated that stigmasterol may be useful in prevention of certain cancers, including ovarian, prostate, breast, and colon cancers. Studies have also indicated that a diet high in phytoesterols may inhibit the absorption of cholesterol and lower serum cholesterol levels by competing for intestinal absorption. Studies with laboratory animals fed stigmasterol found that both cholesterol and sitosterol absorption decreased 23% and 30%, respectively, over a 6-week period. It also possesses potent antioxidant, hypoglycemic and thyroid inhibiting properties.[11]
Potential precursor of boldenone
Being a steroid, stigmasterol is precursor of anabolic steroid boldenone. Boldenone undecylenate is commonly used in veterinary medicine to induce growth in cattle, but it is also one of the most commonly abused anabolic steroids in sports. This led to suspicion that some athletes testing positive for boldenone didn't consume the steroid itself, but rather consumed foods rich in stigmasterol; this turned out not to be the case.[12][13][14]
See also
- Charantin, a stigmasteryl glucoside found in the bitter melon plant.
- Stigmastanol, a closely related phytosterol
- Sitosterol
References
- 1 2 stigmasterol, ChemicalLand21.com
- ↑ "Rosalind Wulzen (b. 1886)". Archives, Manuscripts and Photographs catalog. Smithsonian Institution. Retrieved 14 October 2015.
- ↑ Constituents of Mirabilis jalapa. Siddiqui S., Siddiqui B.S., Adil Q. and Begum S., Fitoterapia, 1990, Volume 61, No. 5, page 471 (abstract)
- ↑ Han JH, Yang YX, Feng MY (2008). "Contents of phytosterols in vegetables and fruits commonly consumed in China". Biomed Environ Sci. 21 (6): 449–453. doi:10.1016/S0895-3988(09)60001-5. PMID 19263798.
- ↑ Sundararaman P, Djerassi C (1977). "A convenient synthesis of progesterone from stigmasterol". J Org Chem. 42 (22): 3633–3634. doi:10.1021/jo00442a044. PMID 915584.
- ↑ "Nova Transcripts: Forgotten Genius". PBS.org. February 6, 2007.
- ↑ "Giants of the Past". lipidlibrary.aocs.org.
- ↑ Kametani T, Furuyama H (1987). "Synthesis of vitamin D3 and related compounds". Med Res Rev. 7 (2): 147–171. doi:10.1002/med.2610070202. PMID 3033409.
- ↑ Hogg, John A. (1992). "Steroids, the steroid community, and Upjohn in perspective: A profile of innovation". Steroids. 57 (12): 593–616. doi:10.1016/0039-128X(92)90013-Y. PMID 1481225.
- ↑ Soy Infocenter (2009). History of Soybean and Soyfoods in Mexico and Central America (1877-2009). ISBN 9781928914211.
- ↑ Panda S, Jafri M, Kar A, Meheta BK (2009). "Thyroid inhibitory, antiperoxidative and hypoglycemic effects of stigmasterol isolated from Butea monosperma". Fitoterapia. 80 (2): 123–126. doi:10.1016/j.fitote.2008.12.002. PMID 19105977.
- ↑ G. Gallina; G. Ferretti; R. Merlanti; C. Civitareale; F. Capolongo; R. Draisci; C. Montesissa (2007). "Boldenone, Boldione, and Milk Replacers in the Diet of Veal Calves: The Effects of Phytosterol Content on the Urinary Excretion of Boldenone Metabolites". J. Agric. Food Chem. 55 (20): 8275–8283. doi:10.1021/jf071097c. PMID 17844992.
- ↑ Ros MM, Sterk SS, Verhagen H, Stalenhoef AF, de Jong N (2007). "Phytosterol consumption and the anabolic steroid boldenone in humans: a hypothesis piloted". Food Addit. Contam. 24 (7): 679–684. doi:10.1080/02652030701216727. PMID 17613052.
- ↑ R. Draisci; R. Merlanti; G. Ferretti; L. Fantozzi; C. Ferranti; F. Capolongo; S. Segato; C. Montesissa (2007). "Excretion profile of boldenone in urine of veal calves fed two different milk replacers". Analytica Chimica Acta. 586 (1–2): 171–176. doi:10.1016/j.aca.2007.01.026. PMID 17386709.