Spermatogonial Stem Cells
Spermatogonial stem cells (SSCs) are a subtype of undifferentiated spermatogonia.
Spermatogonial Stem Cells in the Testis
During foetal development gonocytes develop from primordial germ cells and following this SSCs develop from gonocytes in the testis.[1] SSCs are the early precursor for spermatozoa and are responsible for the continuation of spermatogenesis in adult mammals. The stem cells are capable of dividing into more SSCs which is vital for maintaining the stem cell pool. Alternatively they go on to differentiate into spermatocytes, spermatids and finally spermatozoa.
One SSC is the precursor for multiple spermatozoa and therefore SSCs are much less numerous in the testes than cells undergoing spermatogenesis.
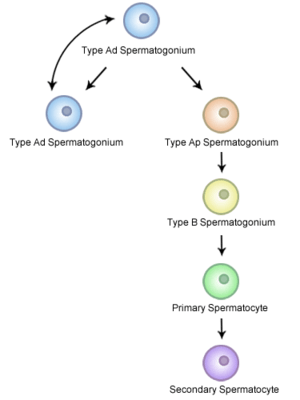
Nomenclature [2]
In Humans
Undifferentiated spermatogonia can be split into 2 groups; A Dark (Ad) and A Pale (Ap)
Ad spermatogonia are reserve stem cells. These cells are capable of dividing to produce more SSCs but usually do not. Ap spermatogonia are actively dividing to maintain the stem cell pool. B1-B4 spermatogonia encompass the differentiating spermatogonia and are no longer considered to be stem cells.
Most research into SSCs has been carried out in rodents. The subtypes of spermatogonia differs between mice and mammals.
In mice
A Single (As) spermatogonia are capable of creating 2 separate daughter SSCs when they divide or the daughter cells can join and form A Paired (Apr) spermatogonia.
Both As and Apr spermatogonia are undifferentiated. Chains of these cells form and are referred to as A Aligned (Aal). Aal spermatogonia differentiate and thus are no longer classed as stem cells. They go on to divide 6 times eventually forming B type spermatogonia.
SSC Niche [3]
The most important somatic cells that support regulation of SSCs are Sertoli cells Various other somatic cells in the interstitial tissue support Sertoli cells such as Leydig cells and peritubular myoid cells therefore indirectly influencing SSCs and the location of their niche.
Spermatogonia stem cells in mammals are found between the basal membrane of the seminiferous tubules and the Sertoli cells. They remain here until the meiotic prophase stage of meiosis. Here the spermatocytes pass through the basal membrane via the sertoli cell barrier.
SSCs stay within their niche where they are encouraged to self renew. When they move past the basal membrane they differentiate due to cell signals.
Paracrine regulation of SSC self-renewal
Self-renewal of spermatogonial stem cells (SSCs) is regulated by local signals.[4] Around 50% of the SSC population undergo self-renewal to maintain stem cell numbers, and the other 50% become committed progenitor cells that will differentiate into spermatozoa during spermatogenesis.[5] Cells present in the testes express molecules that play key roles in the regulation of SSC self-renewal. In mice, Sertoli cells have been shown to secrete Glial cell line-derived neurotrophic factor (GDNF) which has a stimulatory effect on stem cell self-renewal. This factor is thought to be expressed in the peritubular cells in human testes.[1] Fibroblast growth factor (FGF2) is another molecule crucial for the regulation of stem cell renewal and is expressed in Sertoli cells, Leydig cells and germ cells. FGF2 signalling interacts with GDNF to enhance proliferation rate.[1] Chemokine (C-X-C motif) ligand 12 (CXCL12) signaling via its receptor C-X-C chemokine receptor type 4 (CXCR4) is also involved in regulation of SSC fate decisions.CXCL12 is found in Sertoli cells in the basement membrane of the seminiferous tubules in adult mouse testes, and its receptor is expressed in undifferentiated spermatogonial cells.[2]
GDNF and FGF2 are both required to activate the phosphoinositide 3-kinase (PI3K)-Akt pathway, and the mitogen-activated protein kinase/ERK1 kinase1 (MEK) pathway, which potentiate SSC proliferation and survival.[6] CXCL12, FGF2 and GDNF all communicate via a network to mediate SSC functions.[2]
Differentiation of SSCs
Spermatogonial stem cells are the precursors to spermatozoa, which are produced through a series of differentiation steps.[1] This is the alternative SSC outcome to self-renewal. SSCs survive within microenvironments, termed niches, which provide extrinsic stimuli that drive stem cell differentiation or self-renewal.[7] The SSC niche is found in the seminiferous epithelium of mammalian testis, and is primarily constituted of Sertoli and peritubular myoid cells.[2]
There are two primary differentiation stages, the first of which involves the transformation of As (single) spermatogonia into daughter progeny Apr (paired) spermatogonia, which are predestined to differentiate. These can divide further to create Aal (A-aligned) spermatogonia.[1]
The second step involves the production of differentiating A1 spermatogonia from Apr or Aal spermatogonia. These A1 spermatogonia undergo a further five divisions to produce A2, A3, A4, intermediate and type B spermatogonia, which can enter meiosis I.[1]
It takes around 64 days to produce mature spermatozoa from differentiating SSCs, and 100million spermatozoa can be produced each day.[2]
One of the major known substances driving the differentiation of SSCs, and therefore the production of spermatozoa, is Retinoic Acid (RA).[3] There are theories supporting the hypotheses of both an indirect (via Sertoli cells) or a direct pathway.[1]
It is thought that Sertoli cells produce RA through the conversion of circulating retinol to retinal and then finally to RA.[3] Exposure to RA drives cellular differentiation into A1 spermatogonia and is implicated in further meiotic differentiation.[1] As a result of differentiation, the genes required to maintain a SSC state are no longer expressed.[3]
SSC isolation and culture
SSCs have the potential to become increasingly clinically relevant in treating sterility (in vitro spermatogenesis) and preserving fertility prior to gonadotoxic treatments.[8] To this aim, SSCs must be reliably isolated from testicular biopsies for the purposes of e.g. expansion and purification. Current protocols include magnetic-activated cell sorting (MACS) and fluorescence-activated cell sorting (FACS) based on positive SSC cellular markers such as CD90 [9] and FGFR3 [10] in combination with negative markers like CD45.[9] The latter are particularly important in excluding malignant cells from cancer patients’ biopsies.
Once isolated, SSC populations are cultured for the purposes of amplification, characterisation, line maintenance and potentially in vitro spermatogenesis or genomic editing.[11] The main challenges to SSC culturing are the interactions between media substances and the epigenetic makeup that underlies pluripotency and can affect future offspring. Short-term in vitro propagation of these cells has been carried out in Stem-Pro 34 media supplemented by growth factors.[12] Long-term culture of human SSCs is not established yet, however one group reports successful proliferation in feeder cell-free media supplied with growth factors and hydrogel.[13]
SSC transplantation
The first successful SSC transplantation was described in mice in 1994 whereby the procedure restored spermatogenesis fully in an otherwise infertile mouse.[14] These mice were then able to produce viable offspring which opened new exciting doors for future potential therapies in humans.
As cancer treatments are not cancer cell specific and are often gonadotoxic (toxic to the ovaries and the testes), children usually face infertility as a consequence of treatment as there is no established way to preserve their fertility yet, especially in prepubertal boys. Infertility after cancer treatment depends on the type and dosage of treatment but can vary from 17% to 82% of patients.[15] Spermatogonial stem cell therapy (SSCT) has been proposed as a potential method to restore fertility in such cancer survivors who desire to have children later in life. The method has been tested in numerous animal models including non-human primates; Hermann et al.[16] took out and isolated SSCs from prepubertal and adult rhesus macaques before treating them with busulfan (an alkylating agent used in chemotherapy). SSCs were then injected back into the rete testis of the same animal that they were taken from ~10–12 weeks after treatment and spermatogenesis was observed in almost all recipients (16/17). However, these SSCs were difficult to detect which is why further analysis of the ability of descendant sperm to fertilise could not be determined. The viability of embryos fertilised by donor sperm after SSC transplantation needs to be evaluated to truly determine the usefulness of this technique.
Recently, SSC transplantation has also been proposed as a potential method for conservation of endangered species through xenogeneic transplantation. Roe et al. [17] suggested that the reproductive lifespan of such species could be extended by transplanting their germ cells into a domestic host. In their study they used the quail as a model for an exotic species and transplanted SSCs into chicken embryos which successfully colonized the gonadal ridge of the host embryo. This allows the isolation of mature sperm later on in development from the host even after the donor has deceased which can be used in future fertilization and potentially more successful conservation.
References
- 1 2 3 4 5 6 7 8 Phillips, Bart T.; Gassei, Kathrin; Orwig, Kyle E. (2010-05-27). "Spermatogonial stem cell regulation and spermatogenesis". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences. 365 (1546): 1663–1678. doi:10.1098/rstb.2010.0026. ISSN 1471-2970. PMC 2871929
 . PMID 20403877.
. PMID 20403877. - 1 2 3 4 5 Boitani, Carla; Di Persio, Sara; Esposito, Valentina; Vicini, Elena (2016-03-05). "Spermatogonial cells: mouse, monkey and man comparison". Seminars in Cell & Developmental Biology. doi:10.1016/j.semcdb.2016.03.002. ISSN 1096-3634. PMID 26957475.
- 1 2 3 4 de Rooij, Dirk G. (2009-08-01). "The spermatogonial stem cell niche". Microscopy Research and Technique. 72 (8): 580–585. doi:10.1002/jemt.20699. ISSN 1097-0029. PMID 19263493.
- ↑ Kubota, Hiroshi; Avarbock, Mary R.; Brinster, Ralph L. (2004-11-23). "Growth factors essential for self-renewal and expansion of mouse spermatogonial stem cells". Proceedings of the National Academy of Sciences of the United States of America. 101 (47): 16489–16494. doi:10.1073/pnas.0407063101. ISSN 0027-8424. PMC 534530
 . PMID 15520394.
. PMID 15520394. - ↑ de Rooij, Dirk G; Grootegoed, J Anton (1998-12-01). "Spermatogonial stem cells". Current Opinion in Cell Biology. 10 (6): 694–701. doi:10.1016/S0955-0674(98)80109-9.
- ↑ Kanatsu-Shinohara, Mito; Shinohara, Takashi (2013-01-01). "Spermatogonial Stem Cell Self-Renewal and Development". Annual Review of Cell and Developmental Biology. 29 (1): 163–187. doi:10.1146/annurev-cellbio-101512-122353. PMID 24099084.
- ↑ Oatley, Jon M.; Brinster, Ralph L. (2008-01-01). "Regulation of spermatogonial stem cell self-renewal in mammals". Annual Review of Cell and Developmental Biology. 24: 263–286. doi:10.1146/annurev.cellbio.24.110707.175355. ISSN 1081-0706. PMC 4066667
 . PMID 18588486.
. PMID 18588486. - ↑ Galuppo, Andrea Giannotti (2016-12-01). "Spermatogonial stem cells as a therapeutic alternative for fertility preservation of prepubertal boys". Einstein (São Paulo, Brazil). 13 (4): 637–639. doi:10.1590/S1679-45082015RB3456. ISSN 2317-6385. PMID 26761559.
- 1 2 Smith, James F.; Yango, Pamela; Altman, Eran; Choudhry, Shweta; Poelzl, Andrea; Zamah, Alberuni M.; Rosen, Mitchell; Klatsky, Peter C.; Tran, Nam D. (2014-09-01). "Testicular niche required for human spermatogonial stem cell expansion". Stem Cells Translational Medicine. 3 (9): 1043–1054. doi:10.5966/sctm.2014-0045. ISSN 2157-6564. PMID 25038247.
- ↑ von Kopylow, K.; Schulze, W.; Salzbrunn, A.; Spiess, A.-N. (2016-04-01). "Isolation and gene expression analysis of single potential human spermatogonial stem cells". Molecular Human Reproduction. 22 (4): 229–239. doi:10.1093/molehr/gaw006. ISSN 1460-2407. PMID 26792870.
- ↑ Mulder, Callista L.; Zheng, Yi; Jan, Sabrina Z.; Struijk, Robert B.; Repping, Sjoerd; Hamer, Geert; van Pelt, Ans M. M. (2016-09-01). "Spermatogonial stem cell autotransplantation and germline genomic editing: a future cure for spermatogenic failure and prevention of transmission of genomic diseases". Human Reproduction Update. 22 (5): 561–573. doi:10.1093/humupd/dmw017. ISSN 1460-2369. PMID 27240817.
- ↑ Akhondi, Mohammad Mehdi; Mohazzab, Arash; Jeddi-Tehrani, Mahmood; Sadeghi, Mohammad Reza; Eidi, Akram; Khodadadi, Abbas; Piravar, Zeinab (2013-07-01). "Propagation of human germ stem cells in long-term culture". Iranian Journal of Reproductive Medicine. 11 (7): 551–558. ISSN 1680-6433. PMID 24639790.
- ↑ Guo, Ying; Liu, Linhong; Sun, Min; Hai, Yanan; Li, Zheng; He, Zuping (2015-08-01). "Expansion and long-term culture of human spermatogonial stem cells via the activation of SMAD3 and AKT pathways". Experimental Biology and Medicine (Maywood, N.J.). 240 (8): 1112–1122. doi:10.1177/1535370215590822. ISSN 1535-3699. PMID 26088866.
- ↑ Brinster, R. L.; Avarbock, M. R. (1994-11-22). "Germline transmission of donor haplotype following spermatogonial transplantation". Proceedings of the National Academy of Sciences of the United States of America. 91 (24): 11303–11307. ISSN 0027-8424. PMC 45219
 . PMID 7972054.
. PMID 7972054. - ↑ Struijk, Robert B.; Mulder, Callista L.; van der Veen, Fulco; van Pelt, Ans M. M.; Repping, Sjoerd (2013-01-01). "Restoring Fertility in Sterile Childhood Cancer Survivors by Autotransplanting Spermatogonial Stem Cells: Are We There Yet?". BioMed Research International. 2013. doi:10.1155/2013/903142. ISSN 2314-6133. PMC 3581117
 . PMID 23509797.
. PMID 23509797. - ↑ Hermann, Brian P.; Sukhwani, Meena; Winkler, Felicity; Pascarella, Julia N.; Peters, Karen A.; Sheng, Yi; Valli, Hanna; Rodriguez, Mario; Ezzelarab, Mohamed (2012-11-02). "Spermatogonial stem cell transplantation into Rhesus testes regenerates spermatogenesis producing functional sperm". Cell stem cell. 11 (5): 715–726. doi:10.1016/j.stem.2012.07.017. ISSN 1934-5909. PMC 3580057
 . PMID 23122294.
. PMID 23122294. - ↑ Roe, Mandi; McDonald, Nastassja; Durrant, Barbara; Jensen, Thomas (2013-05-01). "Xenogeneic transfer of adult quail (Coturnix coturnix) spermatogonial stem cells to embryonic chicken (Gallus gallus) hosts: a model for avian conservation". Biology of Reproduction. 88 (5): 129. doi:10.1095/biolreprod.112.105189. ISSN 1529-7268. PMC 4013913
 . PMID 23575150.
. PMID 23575150.