Serine C-palmitoyltransferase
| serine C-palmitoyltransferase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 2.3.1.50 | ||||||||
| CAS number | 62213-50-7 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / EGO | ||||||||
| |||||||||
| Serine palmitoyltransferase | |
|---|---|
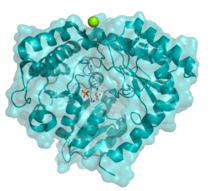 Crystallographic structure of serine palmitoyltransferase from S. paucimobilis. The cofactor PLP is visible in the center.[1] | |
| Identifiers | |
| Symbol | SPT1 |
| PDB | 2JG2 |
| UniProt | Q93UV0 |
| Other data | |
| EC number | 2.3.1.50 |
| serine palmitoyltransferase, long chain base subunit 1 | |
|---|---|
| Identifiers | |
| Symbol | SPTLC1 |
| Alt. symbols | HSN1 |
| Entrez | 10558 |
| HUGO | 11277 |
| OMIM | 605712 |
| RefSeq | NM_006415 |
| UniProt | O15269 |
| Other data | |
| EC number | 2.3.1.50 |
| Locus | Chr. 9 q22.31 |
| serine palmitoyltransferase, long chain base subunit 2 | |
|---|---|
| Identifiers | |
| Symbol | SPTLC2 |
| Entrez | 9517 |
| HUGO | 11278 |
| OMIM | 605713 |
| RefSeq | NM_004863 |
| UniProt | O15270 |
| Other data | |
| EC number | 2.3.1.50 |
| Locus | Chr. 14 q24.3 |
| serine palmitoyltransferase, long chain base subunit 3 | |
|---|---|
| Identifiers | |
| Symbol | SPTLC3 |
| Alt. symbols | C20orf38, SPTLC2L |
| Entrez | 55304 |
| HUGO | 16253 |
| OMIM | 611120 |
| RefSeq | NM_018327 |
| UniProt | Q9NUV7 |
| Other data | |
| EC number | 2.3.1.50 |
| Locus | Chr. 20 p12.1 |
In enzymology, a serine C-palmitoyltransferase (EC 2.3.1.50) is an enzyme that catalyzes the chemical reaction:[2][3]
- palmitoyl-CoA + L-serine CoA + 3-dehydro-D-sphinganine + CO2
Thus, the two substrates of this enzyme are palmitoyl-CoA and L-serine, whereas its 3 products are CoA, 3-dehydro-D-sphinganine, and CO2.[4][5] This reaction is a key step in the biosynthesis of sphingosine which is a precursor of many other sphingolipids.[3]
This enzyme belongs to the family of transferases, specifically those acyltransferases transferring groups other than aminoacyl groups. The systematic name of this enzyme class is palmitoyl-CoA:L-serine C-palmitoyltransferase (decarboxylating). Other names in common use include serine palmitoyltransferase, SPT, 3-oxosphinganine synthetase, and acyl-CoA:serine C-2 acyltransferase decarboxylating. This enzyme participates in sphingolipid metabolism. It employs one cofactor, pyridoxal phosphate.
Structure
Serine C-palmitoyltransferase is a member of the AOS (a-oxoamine synthase) family of PLP-dependent enzymes, which catalyse the condensation of amino acids and acyl-CoA thioester substrates.[6] The human enzyme is a heterodimer consisting of two monomeric subunits known as long chain base 1 and 2 (LCB1/2) encoded by separate genes.[1] The active site of LCB2 contains lysine and other key catalytic residues that are not present in LCB1, which does not participate in catalysis but is nevertheless required for the synthesis and stability of the enzyme.[7]
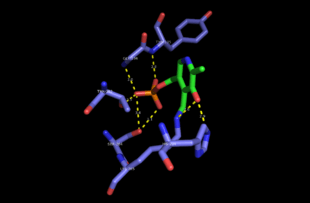
Mechanism
The PLP (pyridoxal 5′-phosphate)-dependent serine C-palmitoyltransferase carries out the first enzymatic step of de novo sphingolipid biosynthesis. The enzyme catalyses a Claisen-like condensation between L-serine and an acyl-CoA thioester (CoASH) substrate (typically C16-palmitoyl) or an acyl-ACP (acyl-carrier protein) thioester substrate, to form 3-ketodihydrosphingosine. Initially PLP cofactor is bound to the active-site lysine via a Schiff base to form the holo-form or internal aldimine of the enzyme. The amine group of L-serine then attacks and displaces the lysine bound to PLP, forming the external aldimine intermediate. Subsequently, deprotonation occurs at the Cα of serine, forming the quinonoid intermediate that attacks the incoming thioester substrate. Following decarboxylation and lysine attack, the product 3-ketodihydrosphingosine is released and catalytically active PLP is reform. This condensation reaction forms the sphingoid base or long-chain base found in all subsequent intermediate sphingolipids and complex sphingolipids in the organism.[3]
Isoforms
A variety of different serine C-palmitoyltransferase isoforms exist across different species. Unlike in eukaryotes, where the enzyme is heterodimeric and membrane bound, bacterial enzymes are homodimers and cytoplasmic. Studies of the isoform of the enzyme found in the Gram-negative bacterium S. paucimobilis were the first to elucidate the structure of the enzyme, revealing that PLP cofactor is held in place by several active site residues including Lys265 and His159.[8] Specifically, the S. paucimobilis isoform features an active-site arginine residue (Arg378) that plays a key role in stabilizing the carboxy moiety of the PLP-L-serine external aldimine intermediate. Similar arginine residues in enzyme homologues (Arg370, Arg390) play analogous roles.[9] Other homologues, such as in Sphingobacterium multivorum, feature the carboxy moiety bound to serine and methionine residues via water in place of arginine.[10] Certain enzyme homologues, such as in S. multivorum as well as B. stolpii, are found to be associated with the inner cell membrane, thus resembling the eukaryotic enzymes.[11] The B. stolpii homologue also features substrate inhibition by palmitoyl-CoA, a feature shared by the yeast and mammalian homologues.[12][13][14]
Disease
HSAN1 (hereditary sensory and autonomic neuropathy type 1) is a genetic disorder caused by mutations in either one of SPTLC1 or SPTLC2, genes encoding the two heterodimeric subunits of the eukaryotic serine C-palmitoyltransferase enzyme.[15][16][17] These mutations have been shown to alter active site specificity, specifically by enhancing the ability of the enzyme to condense L-alanine with the palmitoyl-CoA substrate.[18] This is consistent with elevated levels of deoxysphingoid bases formed by the condensation of alanine with palmitoyl-CoA observed in HSAN1 patients.[19]
Species distribution
Serine C-palmitoyltransferase is expressed in a large number of species from bacteria to humans. The bacterial enzyme is a water-soluble homodimer[2] whereas in eukaryotes the enzyme is a heterodimer which is anchored to the endoplasmic reticulum.[3] Humans and other mammals express three paralogous subunits SPTLC1, SPTLC2, and SPTLC3. It was originally proposed that the functional human enzyme is a heterodimer between a SPTLC1 subunit and a second subunit which is either SPTLC2 or SPTLC3.[20] However more recent data suggest that the enzyme may exist as a larger complex, possibly an octamer, comprising all three subunits.[21]
Structural studies
As of late 2007, two structures have been solved for this class of enzymes, with PDB accession codes 2JG2 and 2JGT.[1]
References
- 1 2 3 Yard, Beverley A.; Carter, Lester G.; Johnson, Kenneth A.; Overton, Ian M.; Dorward, Mark; Liu, Huanting; McMahon, Stephen A.; Oke, Muse; Puech, Daphné (2007-07-27). "The structure of serine palmitoyltransferase; gateway to sphingolipid biosynthesis". Journal of Molecular Biology. 370 (5): 870–886. doi:10.1016/j.jmb.2007.04.086. ISSN 0022-2836. PMID 17559874.
- 1 2 Ikushiro H, Hayashi H, Kagamiyama H (April 2003). "Bacterial serine palmitoyltransferase: a water-soluble homodimeric prototype of the eukaryotic enzyme". Biochim. Biophys. Acta. 1647 (1-2): 116–20. doi:10.1016/S1570-9639(03)00074-8. PMID 12686119.
- 1 2 3 4 Hanada K (June 2003). "Serine palmitoyltransferase, a key enzyme of sphingolipid metabolism". Biochim. Biophys. Acta. 1632 (1-3): 16–30. doi:10.1016/S1388-1981(03)00059-3. PMID 12782147.
- ↑ Brady RN, Di Mari SJ, Snell EE (January 1969). "Biosynthesis of sphingolipid bases. 3. Isolation and characterization of ketonic intermediates in the synthesis of sphingosine and dihydrosphingosine by cell-free extracts of Hansenula ciferri". J. Biol. Chem. 244 (2): 491–6. PMID 4388074.
- ↑ Stoffel W, LeKim D, Sticht G (May 1968). "Biosynthesis of dihydrosphingosine in vitro". Hoppe-Seyler's Z. Physiol. Chem. 349 (5): 664–70. doi:10.1515/bchm2.1968.349.1.664. PMID 4386961.
- ↑ Eliot, Andrew C.; Kirsch, Jack F. (2004-01-01). "Pyridoxal phosphate enzymes: mechanistic, structural, and evolutionary considerations". Annual Review of Biochemistry. 73: 383–415. doi:10.1146/annurev.biochem.73.011303.074021. ISSN 0066-4154. PMID 15189147.
- ↑ Han, Gongshe; Gable, Ken; Yan, Lianying; Natarajan, Mukil; Krishnamurthy, Jayasree; Gupta, Sita D.; Borovitskaya, Anna; Harmon, Jeffrey M.; Dunn, Teresa M. (2004-12-17). "The Topology of the Lcb1p Subunit of Yeast Serine Palmitoyltransferase". Journal of Biological Chemistry. 279 (51): 53707–53716. doi:10.1074/jbc.M410014200. ISSN 0021-9258. PMID 15485854.
- ↑ Shiraiwa, Yuka; Ikushiro, Hiroko; Hayashi, Hideyuki (2009-06-05). "Multifunctional Role of His159in the Catalytic Reaction of Serine Palmitoyltransferase". Journal of Biological Chemistry. 284 (23): 15487–15495. doi:10.1074/jbc.M808916200. ISSN 0021-9258. PMC 2786316
 . PMID 19346561.
. PMID 19346561. - ↑ Lowther, Jonathan; Charmier, Guillaume; Raman, Marine C.; Ikushiro, Hiroko; Hayashi, Hideyuki; Campopiano, Dominic J. (2011-06-23). "Role of a conserved arginine residue during catalysis in serine palmitoyltransferase". FEBS Letters. 585 (12): 1729–1734. doi:10.1016/j.febslet.2011.04.013. ISSN 1873-3468.
- ↑ Ikushiro, Hiroko; Islam, Mohammad Mainul; Okamoto, Akihiro; Hoseki, Jun; Murakawa, Takeshi; Fujii, Shigeru; Miyahara, Ikuko; Hayashi, Hideyuki (2009-10-01). "Structural Insights into the Enzymatic Mechanism of Serine Palmitoyltransferase from Sphingobacterium multivorum". Journal of Biochemistry. 146 (4): 549–562. doi:10.1093/jb/mvp100. ISSN 0021-924X. PMID 19564159.
- ↑ Ikushiro, Hiroko; Islam, Mohammad Mainul; Tojo, Hiromasa; Hayashi, Hideyuki (2007-08-01). "Molecular Characterization of Membrane-Associated Soluble Serine Palmitoyltransferases from Sphingobacterium multivorum and Bdellovibrio stolpii". Journal of Bacteriology. 189 (15): 5749–5761. doi:10.1128/JB.00194-07. ISSN 0021-9193. PMC 1951810
 . PMID 17557831.
. PMID 17557831. - ↑ Gable, Ken; Slife, Harry; Bacikova, Dagmar; Monaghan, Erin; Dunn, Teresa M. (2000-03-17). "Tsc3p Is an 80-Amino Acid Protein Associated with Serine Palmitoyltransferase and Required for Optimal Enzyme Activity". Journal of Biological Chemistry. 275 (11): 7597–7603. doi:10.1074/jbc.275.11.7597. ISSN 0021-9258. PMID 10713067.
- ↑ Hanada, Kentaro; Hara, Tomoko; Nishijima, Masahiro (2000-03-24). "Purification of the Serine Palmitoyltransferase Complex Responsible for Sphingoid Base Synthesis by Using Affinity Peptide Chromatography Techniques". Journal of Biological Chemistry. 275 (12): 8409–8415. doi:10.1074/jbc.275.12.8409. ISSN 0021-9258. PMID 10722674.
- ↑ Lara, Primo N.; Moon, James; Redman, Mary W.; Semrad, Thomas J.; Kelly, Karen; Allen, Jeffrey W.; Gitlitz, Barbara J.; Mack, Philip C.; Gandara, David R. (2015-01-01). "Relevance of platinum sensitivity status in relapsed/refractory extensive stage small cell lung cancer (ES-SCLC) in the modern era: A patient level analysis of SWOG trials". Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. 10 (1): 110–115. doi:10.1097/JTO.0000000000000385. ISSN 1556-0864. PMC 4320001
 . PMID 25490004.
. PMID 25490004. - ↑ Bejaoui, K.; Wu, C.; Scheffler, M. D.; Haan, G.; Ashby, P.; Wu, L.; de Jong, P.; Brown, R. H. (2001-03-01). "SPTLC1 is mutated in hereditary sensory neuropathy, type 1". Nature Genetics. 27 (3): 261–262. doi:10.1038/85817. ISSN 1061-4036. PMID 11242106.
- ↑ Gable, Ken; Han, Gongshe; Monaghan, Erin; Bacikova, Dagmar; Natarajan, Mukil; Williams, Robert; Dunn, Teresa M. (2002-03-22). "Mutations in the Yeast LCB1 and LCB2Genes, Including Those Corresponding to the Hereditary Sensory Neuropathy Type I Mutations, Dominantly Inactivate Serine Palmitoyltransferase". Journal of Biological Chemistry. 277 (12): 10194–10200. doi:10.1074/jbc.M107873200. ISSN 0021-9258. PMID 11781309.
- ↑ Rotthier, Annelies; Auer-Grumbach, Michaela; Janssens, Katrien; Baets, Jonathan; Penno, Anke; Almeida-Souza, Leonardo; Van Hoof, Kim; Jacobs, An; De Vriendt, Els (2010-10-08). "Mutations in the SPTLC2 subunit of serine palmitoyltransferase cause hereditary sensory and autonomic neuropathy type I". American Journal of Human Genetics. 87 (4): 513–522. doi:10.1016/j.ajhg.2010.09.010. ISSN 1537-6605. PMC 2948807
 . PMID 20920666.
. PMID 20920666. - ↑ Gable, Kenneth; Gupta, Sita D.; Han, Gongshe; Niranjanakumari, Somashekarappa; Harmon, Jeffrey M.; Dunn, Teresa M. (2010-07-23). "A Disease-causing Mutation in the Active Site of Serine Palmitoyltransferase Causes Catalytic Promiscuity". Journal of Biological Chemistry. 285 (30): 22846–22852. doi:10.1074/jbc.M110.122259. ISSN 0021-9258. PMC 2906276
 . PMID 20504773.
. PMID 20504773. - ↑ Penno, Anke; Reilly, Mary M.; Houlden, Henry; Laurá, Matilde; Rentsch, Katharina; Niederkofler, Vera; Stoeckli, Esther T.; Nicholson, Garth; Eichler, Florian (2010-04-09). "Hereditary Sensory Neuropathy Type 1 Is Caused by the Accumulation of Two Neurotoxic Sphingolipids". Journal of Biological Chemistry. 285 (15): 11178–11187. doi:10.1074/jbc.M109.092973. ISSN 0021-9258. PMC 2856995
 . PMID 20097765.
. PMID 20097765. - ↑ Hornemann T, Richard S, Rütti MF, Wei Y, von Eckardstein A (December 2006). "Cloning and initial characterization of a new subunit for mammalian serine-palmitoyltransferase". J. Biol. Chem. 281 (49): 37275–81. doi:10.1074/jbc.M608066200. PMID 17023427.
- ↑ Hornemann T, Wei Y, von Eckardstein A (July 2007). "Is the mammalian serine palmitoyltransferase a high-molecular-mass complex?". Biochem. J. 405 (1): 157–64. doi:10.1042/BJ20070025. PMC 1925250
 . PMID 17331073.
. PMID 17331073.