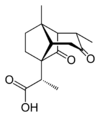
Santonic acid
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
(−)-2,3,3a,4,5,6,7,7a-octahydro-α,3a,5-trimethyl-6,8-dioxo-1,4-methano-1H-indene-1-acetic acid | |||
| Identifiers | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 249878 | ||
| PubChem | 283654 | ||
| |||
| |||
| Properties | |||
| C15H20O4 | |||
| Molar mass | 264.32 g mol−1 | ||
| Density | 1.184 g cm−3[1] | ||
| Melting point | 173 °C (343 °F; 446 K)[1] | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Santonic acid is an organic compound containing both carboxylic acid and ketone functionality.
It was synthesized from santonin by base-mediated hydrolysis of a lactone followed by a multistep rearrangement process by R. B. Woodward.[2]
Unusually for a carboxylic acid, santonic acid does not form hydrogen-bonded dimers in the crystalline phase. Rather, it adopts a polymeric structure, with individual santonic acid molecules linked by intermolecular carboxyl-to-ketone hydrogen bonds.[1]
References
- 1 2 3 A. P. J. Brunskill, H. W. Thompson and R. A. Lalancette (April 1999). "Santonic acid: catemeric hydrogen bonding in a γ,ε-diketo carboxylic acid". Acta Crystallogr. C. 55 (4): 566–568. doi:10.1107/S0108270198014231.
- ↑ Reusch, William (1999). "Base-catalyzed rearrangements". In: Virtual Textbook of Organic Chemistry. Michigan State University. Retrieved September 10, 2008.
This article is issued from Wikipedia - version of the 2/14/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.