Rheological weldability
Rheological weldability (RW) is proposed as a criterion to determine reliably weldability of thermoplastics. In a practical manner, RW of thermoplastics is assessed through their rheological properties: viscosity (η) and activation energy (Ea). According to RW criterion, the lower the viscosity (η) during welding process and the lower the absolute value of activation energy (|Ea|), the better the weldability.[1][2]
Viscosity
The lower the η, the better the RW
Regarding sessile drop technique, wetting is characterized by degree of interfacial contact and quantified via contact angle (θc) of a liquid on a solid surface at equilibrium, as shown in Fig. 1. Interrelation between contact angle and surface tensions at equilibrium is given by the Young equation:[3]
where
- = Solid-Gas surface tension,
- = Solid-Liquid surface tension,
- = Liquid-Gas surface tension,
- = Contact angle.
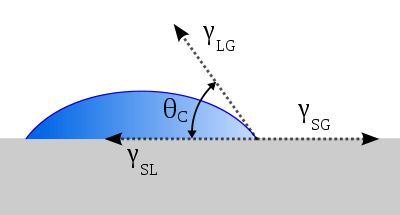
For perfectly good wetting, contact angle (θc) at equilibrium should be a minimum. However, it is valid only at equilibrium, and rate of the equilibrium depends on the balance between driving force of wetting and viscosity of the liquid. In the case of polymer melts, viscosity can be very high and it may take a long time to reach the equilibrium contact angle (dynamic contact angle is likely higher than the contact angle at equilibrium).
Consequently, for the evaluation of weldability, viscosity of molten thermoplastics (polymer melts) have to be taken into account since welding is a rapid process. It can be said that the lower the viscosity during welding process (at welding temperature and pressure), the better the weldability.
Recalling that viscosity (η) decreases with increasing temperature (T) and shear rate () for most polymer melts, weldability is better where temperature and shear rate (movement) are higher within all cross-section of welding region.[1][2]
Activation energy
The lower the |Ea|, the better the RW
During operation of a welding process, soften or molten portion of thermoplastics (polymer articles) is able to flow through the interface. Smaller amount of flow causes smaller diffusion at the interface and lower weld strength. In order for a polymer melt to flow, macromolecular chain segments must be able to move. When the chain segments obtain sufficient thermal energy to overcome energy barrier, they can move readily. The energy barrier is called as activation energy (Ea). It can be said that if a polymer’s absolute value of activation energy (|Ea|) is lower, its weldability becomes better.
|Ea| values of such polymers as PVC decrease with increasing shear rate (), implying better weldability where shear rate (movement) are higher within all cross-section of welding region.[1][2]
Using viscosity-shear rate () data at various temperatures for a polymer, activation energy (Ea) can be calculated via Arrhenius equation:[4][5][6]
where
- η is viscosity of molten polymer,
- C is pre-exponential factor,
- R is universal gas constant,
- T is temperature (Kelvin).
How to calculate absolute value of activation energy (|Ea|) by taking the natural logarithm of Arrhenius equation can be readily learned elsewhere (see Arrhenius equation).
See also
References
- 1 2 3 O.Balkan, H.Demirer, A.Ezdesir, H.Yildirim (2008). "Effects of welding procedures on mechanical and morphological properties of hot gas butt welded PE, PP, and PVC sheets". Polymer Engineering and Science. 48: 732. doi:10.1002/pen.21014. ISSN 1548-2634.
- 1 2 3 O.Balkan, A.Ezdesir (October 15–17, 2008). Rheological Weldability of Polymers. 12. International Materials Symposium (12.IMSP) Denizli. p. 1046.
- ↑ Young, T. (1805). "An Essay on the Cohesion of Fluids". Phil. Trans. R. Soc. Lond. 95: 65–87. doi:10.1098/rstl.1805.0005.
- ↑ Arrhenius, S.A. (1889). "Über die Dissociationswärme und den Einflusß der Temperatur auf den Dissociationsgrad der Elektrolyte". Z. Phys. Chem. 4: 96–116.
- ↑ Arrhenius, S.A. (1889). "Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren". ibid. 4: 226–248.
- ↑ Laidler, K. J. (1987) Chemical Kinetics,Third Edition, Harper & Row, p.42