Rapid sequence induction
| Rapid sequence induction/intubation | |
|---|---|
| Intervention | |
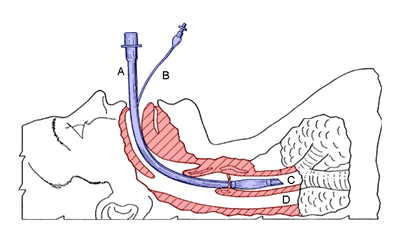 Diagram showing the result of a successful RSI: an endotracheal tube (blue) inserted into the trachea (C), protecting the lungs from regurgitation through the esophagus (D). | |
| eMedicine | 80222 |
In advanced airway management, rapid sequence induction (RSI) - also described as rapid sequence intubation or as rapid sequence induction and intubation (RSII) - is a special process for tracheal intubation that is used where the patient is at a high risk of pulmonary aspiration. It differs from other forms of general anesthesia induction in that artificial ventilation is generally not provided from the time the patient stops breathing (when drugs are given) until after intubation has been achieved. This minimises insufflation of air into the patient's stomach, which might otherwise provoke regurgitation.
"Classic" RSI involves pre-filling the patient's lungs with a high concentration of oxygen gas, followed by applying cricoid pressure, administering rapid-onset hypnotic and neuromuscular-blocking drugs that induce prompt unconsciousness and paralysis, inserting an endotracheal tube with minimal delay, and then releasing the cricoid pressure. "Modified" RSI refers to changes that deviates from the classic pattern, usually to reduce acidosis or improve oxygenation, but at the expense of increased regurgitation risk; examples of modifications include giving ventilations before the tube has been placed, or not using cricoid pressure.
The procedure is used where general anesthesia must be induced before the patient has had time to fast long enough to empty the stomach; where the patient has a condition that makes aspiration more likely during induction of anesthesia, regardless of how long they have fasted (such as gastroesophageal reflux disease or advanced pregnancy); or where the patient has become unable to protect their own airway even before anesthesia (such as after a traumatic brain injury).
The induction drugs traditionally used for RSI have short durations of action, wearing off after only minutes. This confers a degree of fault tolerance on the procedure when it is used in elective or semi-elective settings: if intubation is unsuccessful, and if the clinical condition allows it, the procedure may be abandoned and the patient should regain the ability to protect their own airway sooner than would be the case under routine methods of induction. Conversely, in emergency settings where the patient's condition does not allow for them to be woken up immediately, a failed intubation under RSI places them at very high risk for respiratory compromise.
Common medications
Commonly used medications during a rapid sequence intubation:
Sedation
- Lidocaine (Please note that lidocaine is used to treat elevated intracranial pressure and is not a sedative.)
- Midazolam
- Ketamine
- Fentanyl
- Thiopentone
- Propofol
- Etomidate
Paralytics
Other medications
- Atropine — For patients where bradycardia is a concern
- Metaraminol or ephedrine, where hypotension (low blood pressure) may occur secondary to the sedating drugs
Technique
Rapid sequence intubation refers to the pharmacologically induced sedation and neuromuscular paralysis prior to intubation of the trachea. The technique is a quicker form of the process normally used to induce general anesthesia. A useful framework for describing the technique of RSI is the "seven Ps".[1]
Preparation
The patient is assessed to predict the difficulty of intubation. Continuous physiological monitoring such as ECG and pulse oximetry is put on the patient. The equipment and drugs for the intubation are planned, including the endotracheal tube size, the laryngoscope size, and drug dosage. Drugs are prepared in syringes. Intravenous access is obtained to deliver the drugs, usually by placing one or two IV cannulae.
Preoxygenation
The aim of preoxygenation is to replace the nitrogen that forms the majority of the functional residual capacity with oxygen. This provides an oxygen reservoir that will delay the depletion of oxygen in the absence of ventilation (after paralysis) for up to 8 minutes (to an oxygen saturation of 90%) in the healthy adult.[2] This time will be significantly reduced in obese patients, ill patients and children. Preoxygenation is usually performed by giving 100% oxygen via a tightly fitting face mask. Preoxygenation or a maximum of eight deep breaths over 60 seconds results in blood oxygenation is not different from that of quiet breathing volume for 3 minutes.[3]
Newer methods of pre oxygenation include the use of a nasal cannula placed on the patient at 15 LPM at least 5 minutes prior to the administration of the sedation and paralytic drugs. High flow nasal oxygen has been shown to flush the nasopharynx with oxygen, and then when patients inspire they inhale a higher percentage of inspired oxygen. Small changes in FiO2 create dramatic changes in the availability of oxygen at the alveolus, and these increases result in marked expansion of the oxygen reservoir in the lungs prior to the induction of apnea. After apnea created by RSI the same high flow nasal cannula will help maintain, or even increase, oxygen saturation during efforts securing the tube (oral intubation). The use of nasal oxygen during pre-oxygenation and continued during apnea can prevent hypoxia before and during intubation, even in extreme clinical cases.[4]
Pretreatment
Pretreatment consists of the medications given to specific groups of high-risk patients 3 minutes before the paralysis stage with the aim of protecting the patient from the adverse effects of introducing the laryngoscope and endotracheal tube. Intubation causes increased sympathetic activity, an increase in intracranial pressure and bronchospasm. Patients with reactive airway disease, increased intracranial pressure, or cardiovascular disease may benefit from pretreatment. Two common medications used in the pretreatment of RSI include Lidocaine and Atropine. Lidocaine has the ability to suppress the cough reflex which in turn may mitigate increased intracranial pressure. For this reason Lidocaine is commonly used as a pretreatment for trauma patients who are suspected of already having an increase in intracranial pressure. Although there is not yet definitive evidence to support this, if proper dosing is used it is safe. The typical dose is 1.5 mg/kg IV given three minutes prior to intubation.[5] Atropine may also be used as a premedication agent in pediatrics to prevent bradycardia caused by hypoxia, laryngoscopy, and succinylcholine. Atropine is parasympathetic blocker. The common premedication dose for atropine is 0.01-0.02 mg/kg.
Paralysis with induction
With standard intravenous induction of general anesthesia, the patient typically receives an opioid, and then a hypnotic medication. Generally the patient will be manually ventilated for a short period of time before a neuromuscular blocking agent is administered and the patient is intubated. During rapid sequence induction, the person still receives an IV opioid. However, the difference lies in the fact that the induction drug and neuromuscular blocking agent are administered in rapid succession with no time allowed for manual ventilation.
Commonly used hypnotics include thiopental, propofol and etomidate. Commonly used neuromuscular blocking agents used include succinylcholine and rocuronium.[6] The neuromuscular blocking agents paralyze all of the skeletal muscles, most notably and importantly in the oropharynx, larynx, and diaphragm. Opioids such as fentanyl may be given to attenuate the responses to the intubation process (accelerated heart rate and increased intracranial pressure). This is supposed to have advantages in patients with ischemic heart disease and those with brain injury (e.g. after traumatic brain injury or stroke). Lidocaine is also theorized to blunt a rise in intracranial pressure during laryngoscopy, although this remains controversial and its use varies greatly. Atropine may be used to prevent a reflex bradycardia from vagal stimulation during laryngoscopy, especially in young children and infants. Despite their common use, such adjunctive medications have not been demonstrated to improve outcomes.[7]
Positioning
Positioning involves bringing the axes of the mouth, pharynx and larynx into alignment. The term used to describe this position is called the "sniffing" position. The sniffing position is achieved by placing a rolled towel underneath the head and neck, effectively extending the head and flexing the neck. You are at proper alignment when the ear is inline with the sternum.[8]
The Sellick's maneuver, or cricoid pressure, may be used to occlude the esophagus with the goal of preventing aspiration.
Placement of tube
During this stage, laryngoscopy is performed to visualize the glottis. The endotracheal tube is then passed in between the vocal cords, and a cuff is inflated around the tube to hold it in place and prevent aspiration of stomach contents.
The position of the tube in the trachea can be confirmed in a number of ways, including observing increasing end tidal carbon dioxide, auscultation of both lungs and stomach, chest movement, and misting of the tube.
Postintubation management
Malpositioning of the endotracheal tube (in a bronchus, above the glottis, or in the esophagus) should be excluded by confirmation of end tidal CO2, auscultation and observation of bilateral chest rise.
One important difference between RSI and routine tracheal intubation is that the practitioner does not typically manually assist the ventilation of the lungs after the onset of general anesthesia and cessation of breathing, until the trachea has been intubated and the cuff has been inflated.[9]
Additional considerations
Age can play a role in whether or not the procedure is warranted, and is commonly needed in younger persons.[10] The clinician that performs RSI must be skilled in tracheal intubation and also in bag valve mask ventilation. Alternative airway management devices must be immediately available, in the event the trachea cannot be intubated using conventional techniques. Such devices include the combitube and the laryngeal mask airway. Invasive techniques such as cricothyrotomy must also be available in the event of inability to intubate the trachea by conventional techniques.
RSI is mainly used to intubate patients at high risk of aspiration, mostly due to a full stomach as commonly seen in a trauma setting. Bag ventilation causes distention of stomach which can induce vomiting, so this phase must be quick. The patient is given a sedative and paralytic agent, usually midazolam / suxamethonium / propofol and intubation is quickly attempted with minimal or no manual ventilation. The patient is assessed for predictable intubation difficulties. Laryngoscope blades and endotracheal tubes smaller than would be used in a non-emergency setting are selected.
If the patient on initial assessment is found to have a difficult airway, RSI is contraindicated since a failed RSI attempt will leave no option but to ventilate the patient on bag and mask which can lead to vomiting. For these challenging cases, awake fiberoptic intubation is usually preferred.
Controversy
Since the introduction of RSI, there has been controversy regarding virtually every aspect of this technique, including:[11]
- choice of intravenous hypnotic agents as well as their dosage and timing of administration
- dosage and timing of administration of neuromuscular blocking agents
- avoidance of manual ventilation before tracheal intubation
- optimal position and whether the head-up, head-down, or horizontal supine position is the safest for induction of anesthesia in full-stomach patients
- application of cricoid pressure, which is also referred to as the Sellick maneuver.
References
- ↑ Cooper, Angus. "Rapid Sequence Intubation - A guide for assistants" (PDF). Scottish Intensive Care Society Education. NHS - Education for Scotland. Retrieved 31 March 2013.
- ↑ Benumof, J. L.; Dagg, R.; Benumof, R. (1997). "Critical hemoglobin desaturation will occur before return to an unparalyzed state following 1 mg/kg intravenous succinylcholine". Anesthesiology. 87 (4): 979–982. doi:10.1097/00000542-199710000-00034. PMID 9357902.
- ↑ http://anesthesiageneral.com/preoxygenation/
- ↑ http://www.epmonthly.com/archives/features/no-desat-/
- ↑ Hampton, J. P. (2011). Rapid-sequence intubation and the role of the emergency department pharmacist. American Journal Of Health-System Pharmacy, 68(14), 1320-1330. doi:10.2146/ajhp100437
- ↑ Pousman, RM (2000). "Rapid Sequence Induction for Prehospital Providers". The Internet Journal of Emergency and Intensive Care Medicine. 4 (1).
- ↑ Neilipovitz, DT; Crosby, ET (2007). "No evidence for decreased incidence of aspiration after rapid sequence intubation". Canadian Journal of Anesthesia. 54 (9): 748–64. doi:10.1007/BF03026872. PMID 17766743.
- ↑ Nancy Caroline: Emergency Care in the Streets 7th Ed. Jones & Bartlett Learning. 2013. p. 780.
- ↑ Stone DJ and Gal TJ (2000). "Airway management". In Miller, RD. Anesthesia, Volume 1 (5th ed.). Philadelphia: Churchill Livingstone. pp. 1414–51. ISBN 978-0-443-07995-5.
- ↑ Warner KJ, Sharar SR, Copass MK, Bulger EM (April 2009). "Prehospital management of the difficult airway: a prospective cohort study". The Journal of Emergency Medicine. 36 (3): 257–65. doi:10.1016/j.jemermed.2007.10.058. PMID 18439793. Retrieved 2012-06-28.
- ↑ El-Orbany, MI; Connolly, LA (2010). "Rapid Sequence Induction and Intubation: Current Controversy" (PDF). Anesthesia & Analgesia. 110 (5): 1318–25. doi:10.1213/ANE.0b013e3181d5ae47.