VSV-EBOV
| Vaccine description | |
|---|---|
| Target disease | Ebola virus |
| Type | Recombinant Vector |
| ChemSpider | none |
Vesicular Stomatitis Virus-Ebola Virus vaccine — known as VSV-EBOV — is an experimental vaccine for protecting against Ebola virus disease. It was created by scientists at the National Microbiology Laboratory in Winnipeg, Canada.[1][2] The Public Health Agency of Canada owns the vaccine and the patent on it, and licensed it to a small company called NewLink Genetics in 2010 so that the vaccine could be developed, approved by regulatory authorities, and made available for use; NewLink in turn licensed the rights to Merck in 2014.
VSV-EBOV is a recombinant, replication-competent vaccine.[3] It consists of a vesicular stomatitis virus, which has been genetically engineered to express Ebola glycoproteins so as to provoke an immune response against the complete Ebola virus.[4] The vaccine variant known as rVSV-ZEBOV, rVSV-ZEBOV-GP, VSVΔG-ZEBOV, or BPSC1001 expresses glycoproteins of the Zaire ebolavirus or ZEBOV, the species causing the highest mortality rate among the ebolaviruses, while rVSV-MARV or rVSV-MARV-GP expresses those of the closely related Marburg filovirus or MARV. As of January 2016, phase III clinical trials were ongoing.[5]
Medical use
VSV-EBOV is a candidate vaccine against Ebola virus disease.[6]
Side effects
In Phase I trials, side effects were mild to moderate, including fever, arthritis and associated pain lasting a median of 8 days.[7][8]
Physical and chemical properties
VSV-EBOV is a live, attenuated recombinant vesicular stomatitis virus in which the gene for the native envelope glycoprotein is replaced with that from Ebola virus Zaire, Kikwit 1995 strain.[6][7][9]Manufacturing of the vaccine for the Phase I trial was done by IDT Biologika.[10][11] Manufacturing of vaccine for the Phase III trial was done by Merck, using cells from African green monkeys, which Merck already used to make its RotaTeq vaccine against rotavirus.[12][13]
History
Scientists working for the Public Health Agency of Canada (PHAC) created the vaccine, and PHAC applied for a patent in 2003.[14][15]From 2005-2009, three animal trials on the virus were published, all of them funded by the Canadian and U.S. governments.[11] In 2005, a single intramuscular injection of the EBOV or MARV vaccine was found to induce completely protective immune responses in nonhuman primates (crab-eating macaques) against corresponding infections with the otherwise typically lethal EBOV or MARV.[16][17]
In 2010 PHAC licensed the intellectual property on the vaccine to a small U.S. company called Bioprotection Systems, which was a subsidiary of NewLink Genetics; Newlink had funding the U.S. Defense Threat Reduction Agency to develop vaccines.[14][18][19]
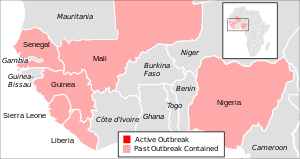
In December 2013, the largest-ever Ebola epidemic started in West Africa, specifically, in Guinea.[20] In September or October 2014, Newlink formed a steering committee among the interested parties, including PHAC, the NIH, and the WHO, to plan the clinical development of the vaccine.[21][22]
In October 2014 NewLink Genetics began a Phase I clinical trial of rVSV-ZEBOV on healthy human subjects to evaluate the immune response, identify any side effects and determine the appropriate dosage.[18][23][24] Phase I trials took place in Gabon, Kenya, Germany, Switzerland, the US, and Canada.[25][26] In November 2014 NewLink exclusively licensed rights to the vaccine to Merck.[27]
In March 2015 a Phase II clinical trial and a Phase III started in Guinea at the same time; the Phase II trial focused on frontline health workers, while the Phase III trial was a ring vaccination in which close contacts of people who contracted Ebola virus were vaccinated with VSV-EBOV.[28] Preliminary results were reported in July.[29] In the same report, the WHO communicated that the control arm of the trial was dropped and the trial would expand.[30] Médecins Sans Frontières, who helped support the study, are recommending that all contacts of new Ebola patients and frontline workers receive the vaccine: "Even if the sample size is quite small and more research and analysis is needed, the enormity of the public health emergency should lead us to continue using this vaccine right now... Replication of a targeted approach focusing on those most at risk of infection should therefore happen immediately and we urge governments in affected countries to start using this vaccine as soon as they can within the framework of the existing trial."[31]
In January 2016 the GAVI Alliance signed an agreement with Merck under which Merck agreed to provide VSV-EBOV vaccine for future outbreaks of Ebola and GAVI paid Merck five million dollars; Merck will use the funds to complete clinical trials and obtain regulatory approval. As of that date Merck had submitted an application to the World Health Organization through their Emergency Use Assessment and Listing (EUAL) program to allow for use of the vaccine in the case of another epidemic.[5]Nearly 800 people were ring vaccinated with VSV-EBOV when another Ebola outbreak occurred in Guinea in March 2016.[32]
See also
References
- ↑ "Canada offers experimental Ebola vaccine VSV-EBOV to West Africa". CBC News. Aug 12, 2014.
- ↑ "Canada's experimental Ebola vaccine: How does it work?". CTV News. August 13, 2014.
- ↑ Marzi, Andrea; et al. "Vesicular Stomatitis Virus–Based Ebola Vaccines With Improved Cross-Protective Efficacy". Journal of Infectious Diseases. 204 (suppl 3): S1066–S1074. doi:10.1093/infdis/jir348. Retrieved 31 July 2015.
- ↑ http://america.aljazeera.com/articles/2015/3/11/why-were-still-waiting-on-an-ebola-vaccine.html
- 1 2 Hirshler, Ben; Kelland, Kate (20 Jan 2016). "Vaccines alliance signs $5 million advance deal for Merck's Ebola shot". Retrieved 20 Jan 2016.
- 1 2 Choi WY; et al. (Jan 2015). "Progress of vaccine and drug development for Ebola preparedness". Clin Exp Vaccine Res. 4 (1): 11–6. doi:10.7774/cevr.2015.4.1.11. PMID 25648233.
- 1 2 Martínez-Romero C, García-Sastre A (2015). "Against the clock towards new Ebola virus therapies". Virus Res. 209: 4–10. doi:10.1016/j.virusres.2015.05.025. PMID 26057711.
- ↑ Miriam Shuchman for the Lancet World Report. May 12, 2015 Ebola vaccine trial in west Africa faces criticism
- ↑ Regules JA; et al. (Apr 2015). "A Recombinant Vesicular Stomatitis Virus Ebola Vaccine - Preliminary Report". N Engl J Med.: 150414100104004. doi:10.1056/NEJMoa1414216. PMID 25830322.
- ↑ Hôpitaux Universitaires de Genève FAQs about the context of this clinical trial: Question 22
- 1 2 The strange tale of Canada’s ebola vaccine: Walkom. Commercial rights to a vaccine worth $50 million were sold to a U.S. middleman for $205,000. By Thomas Walkom, The Star, Nov 25 2014
- ↑ Carly Helfand for FierceVaccine. November 21, 2014 NewLink, Merck sign Ebola vaccine licensing pact
- ↑ Zachary Brennan for BioPharma Reporter. Nov 25, 2014. Merck to manufacture NewLink Ebola vaccine in-house
- 1 2 Denise Grady for the New York Times. October 23, 2014 Ebola Vaccine, Ready for Test, Sat on the Shelf
- ↑ Published PCT Application WO2004011488: Recombinant vesicular stomatitis virus vaccines for viral hemorrhagic fevers, claiming priority to US provisional patent application serial number 60/398,552 filed on July 26, 2003.
- ↑ Sylvain Baize (2005). "A single shot against Ebola and Marburg virus". Nature Medicine. 11 (7): 720–721. doi:10.1038/nm0705-720.
- ↑ Steven M. Jones with thirteen others (2005). "Live attenuated recombinant vaccine protects nonhuman primates against Ebola and Marburg viruses". Nature Medicine. 11 (7): 786–790. doi:10.1038/nm1258. PMID 15937495.
- 1 2 "Ebola outbreak: 1st human trials of Canadian vaccine start in U.S.". CBC News. Oct 13, 2014.
- ↑ Redacted License Agreement
- ↑ "Origins of the 2014 Ebola epidemic". World Health Organization. January 2015. Retrieved Oct 8, 2016.
- ↑ Patricia Van Arnum for DCAT. Oct 21, 2014 Pharmaceutical companies join the effort to develop and produce vaccines and treatments for the Ebola virus
- ↑ Helen Branswell (Oct 8, 2014). "Canadian Ebola vaccine safety trials move ahead, NewLink Genetics says". CBC News. Retrieved Oct 21, 2014.
- ↑ "Canadian Ebola vaccine license holder moving ahead with safety trials". Canadian Press. Oct 8, 2014. Retrieved 13 October 2014.
- ↑ "A Phase 1 Randomized, Double-Blind, Placebo Controlled, Dose-Escalation Study to Evaluate the Safety and Immunogenicity of Prime-Boost VSV Ebola Vaccine in Healthy Adults". US NIAID. Oct 9, 2014. Retrieved 13 October 2014.
- ↑ Hôpitaux Universitaires de Genève FAQs about the context of this clinical trial: Question 10
- ↑ "Table of vaccine clinical trials". World Health Organization. Retrieved 2016-10-14.
- ↑ Staff, Genetic Engineering News. Nov 24, 2014 Merck & Co. Licenses NewLink's Ebola Vaccine Candidate
- ↑ WHO and MSF, 17 July 17, 2015. Q&A on trial of Ebola Virus Disease vaccine in Guinea
- ↑ James Gallagher (31 July 2015). "Ebola vaccine is 'potential game-changer'". BBC News Health. UK: BBC. Retrieved 30 July 2015.
- ↑ Henao-Restrepo, Ana Maria; et al. (31 July 2015). "Efficacy and effectiveness of an rVSV-vectored vaccine expressing Ebola surface glycoprotein: interim results from the Guinea ring vaccination cluster-randomised trial" (PDF). The Lancet. doi:10.1016/S0140673615611175. Retrieved 31 July 2015.
- ↑ http://www.msf.org/article/ebola-getting-closer-ebola-vaccine
- ↑ "WHO coordinating vaccination of contacts to contain Ebola flare-up in Guinea". World Health Organization. March 2016. Retrieved 2016-05-14.
Further reading
- "Scientists hail '100% effective' Ebola vaccine - National Library of Medicine". PubMed Health. National Institute of Health. Retrieved 21 July 2016.
- Marzi, Andrea; Robertson, Shelly J.; Haddock, Elaine; Feldmann, Friederike; Hanley, Patrick W.; Scott, Dana P.; Strong, James E.; Kobinger, Gary; Best, Sonja M.; Feldmann, Heinz (14 August 2015). "VSV-EBOV rapidly protects macaques against infection with the 2014/15 Ebola virus outbreak strain". Science. 349 (6249): 739–742. doi:10.1126/science.aab3920. ISSN 0036-8075. Retrieved 21 July 2016.