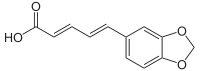
Piperic acid
 | |
| Names | |
|---|---|
| IUPAC name
(2E,4E)-5-(3,4-methylenedioxyphenyl)-2,4-pentadienoic acid | |
| Identifiers | |
| 136-72-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:37316 |
| ChEMBL | ChEMBL332122 |
| ChemSpider | 4521337 |
| EC Number | 226-118-4 |
| MeSH | C017637 |
| PubChem | 5370536 |
| |
| |
| Properties | |
| C12H10O4 | |
| Molar mass | 218.21 g·mol−1 |
| Boiling point | decomposes |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
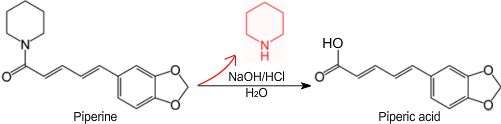
Piperic acid is a chemical often obtained by the base-hydrolysis of the alkaloid piperine[1] from black pepper,[2] followed by acidification of the corresponding salt.[3] Piperic acid is an intermediate in the synthesis of other compounds such as piperonal, and as-such may be used to produce fragrances, perfumes flavorants and drugs as well as other useful compounds.
Preparation
Piperic acid can be prepared from the commercially-available alkaloid piperine, a cyclic amide containing a piperidine group, by reacting it with a hydroxide such as potassium hydroxide, then acidifying the formed piperate salt with hydrochloric acid or another acid. The toxic compound piperidine is given off during the base-hydrolysis of piperine and as-such, safety precautions should be taken.
Reactions
Reaction of piperic acid with strong oxidizers such as potassium permanganate or ozone, or a halogen such as bromine followed by sodium hydroxide causes oxidative cleavage of the double-bonds, yielding piperonal and piperonylic acid.[4][5] Piperonal has many uses in industry and is itself a precursor to a good subsection of other chemicals. On reduction with sodium amalgam piperic acid forms α- and β-dihydropiperic acid, C12H12O4, and the latter can take up two further atoms of hydrogen to produce tetrahydropiperic acid.
See also
References
- ↑ Paul M. Dewick. (2009). Medicinal natural products : a biosynthetic approach. Chichester: A John Wiley & Sons. p. 327. ISBN 978-0-470-74167-2.
- ↑ http://chestofbooks.com/health/aromatherapy/The-Volatile-Oils-Vol1/Heliotropin.html
- ↑ http://www.erowid.org/archive/rhodium/chemistry/3base/piperonal.pepper/degradation.piperine/index.html
- ↑ http://www.freepatentsonline.com/5095128.html, Preparation process for piperonal, US Patent No. 5095128
- ↑ http://www.erowid.org/archive/rhodium/chemistry/3base/piperonal.pepper/cleavage.piperic_acid/index.html