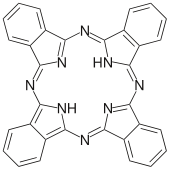
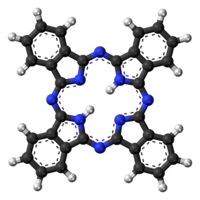
Phthalocyanine
 | |
 | |
| Names | |
|---|---|
| Other names
Phthalocyanin | |
| Identifiers | |
| 574-93-6 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4445497 |
| ECHA InfoCard | 100.008.527 |
| PubChem | 5282330 |
| |
| |
| Properties | |
| C32H18N8 | |
| Molar mass | 514.55 g·mol−1 |
| Hazards | |
| S-phrases | S22 S24/25 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Phthalocyanine is an intensely blue-green-coloured aromatic macrocyclic compound that is widely used in dyeing.[2][3][4] Phthalocyanines form coordination complexes with most elements of the periodic table. These complexes are also intensely colored and also are used as dyes or pigments.
Properties
Unsubstituted phthalocyanine, abbreviated H
2Pc, and many of its complexes have very low solubility in organic solvents. Benzene at 40 °C dissolves less than a milligram of H
2Pc or CuPc per litre. H
2Pc or CuPc dissolve easily in sulfuric acid due to the protonation of the nitrogen atoms bridging the pyrrole rings. Many phthalocyanine compounds are thermally very stable, do not melt but can be sublimed, CuPc sublimes at >500 °C under inert gases (nitrogen, CO
2). Substituted phthalocyanine complexes often have much higher solubility. They are less thermally stable and often can not be sublimed. Unsubstituted phthalocyanines strongly absorb light between 600 and 700 nm, thus these materials are blue or green. Substitution can shift the absorption towards longer wavelengths, changing the color from pure blue to green to colorless (when the absorption is in the near infrared).
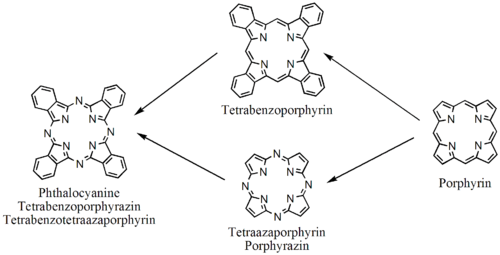
Phthalocyanines are structurally related to other macrocyclic pigments, especially the porphyrins. Both feature four pyrrole-like subunits linked to form a 16-membered ring. The pyrrole-like rings within H
2Pc are closely related to isoindole. Both porphyrins and phthalocyanines function as planar tetradentate dianionic ligands that bind metals through four inwardly projecting nitrogen centers. Such complexes are formally derivatives of Pc2−
, the conjugate base of H
2Pc.
Many derivatives of the parent phthalocyanine are known, where either carbon atoms of the macrocycle are exchanged for nitrogen atoms or where the hydrogen atoms of the ring are substituted by functional groups like halogens, hydroxy, amino, alkyl, aryl, thiol, alkoxy, nitro, etc. groups.
History
An unidentified blue compound, which is now known to be metal-free phthalocyanine, was first reported in 1907.[6] In 1927, Swiss researchers accidentally synthesized copper phthalocyanine, copper naphthalocyanine, and copper octamethylphthalocyanine in an attempted conversion of o-dibromobenzene into phthalonitrile. They remarked on the enormous stability of these complexes but did not further characterize these blue complexes.[7] In the same year, copper phthalocyanine was also discovered at Scottish Dyes, Ltd., Grangemouth, Scotland (later ICI).[8]
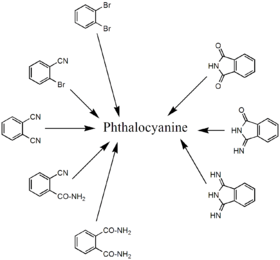
Synthesis
Phthalocyanine forms upon heating phthalic acid derivatives that contain nitrogen functional groups. Classical precursors are phthalonitrile and diiminoisoindole. In the presence of urea, the heating of phthalanhydride is a useful route to H2Pc. These reactions are more efficient in the presence of metal salts. Other precursors include o-cyanobenzamide and phthalimide. Several of these starting materials are shown in the figure below (right side). Using such methods, approximately 57000 tons of various phthalocyanines were produced in 1985.[9]
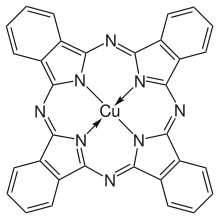 |  |  | |
Halogenated and sulfonated derivatives of copper phthalocyanines are commercially important. Such compounds are prepared by treating CuPc with chlorine, bromine or oleum.
Applications
Approximately 25% of all artificial organic pigments are phthalocyanine derivatives.[9] Copper phthalocyanine (CuPc) dyes are produced by introducing solubilizing groups, such as one or more sulfonic acid functions. These dyes find extensive use in various areas of textile dyeing (Direct dyes for cotton), for spin dyeing and in the paper industry. Direct blue 86 is the sodium salt of CPC-sulfonic acid whereas direct blue 199 is the quaternary ammonium salt of the CPC-sulfonic acid. The quaternary ammonium salts of these sulfonic acids are used as solvent dyes because of their solubility in organic solvents, e.g. Solvent Blue 38 and Solvent Blue 48. The dye derived from cobalt phthalocyanine and an amine is Phthalogen Dye IBN. 1,3-Diiminoisoindolene, the intermediate formed during phthalocyanine manufacture, used in combination with a copper salt affords the dye GK 161.
All major artists' pigment manufacturers produce variants of copper phthalocyanine, designated color index PB15 (blue) and color indexes PG7 and PG36 (green).
Phthalocyanine is also commonly used as a dye in the manufacture of high-speed CD-R media. Most brands of CD-R use this dye except Taiyo Yuden (now CMC Pro) and Verbatim DataLife (which use cyanine and azo dyes, respectively).
Niche applications
Metal phthalocyanines have long been examined as catalysts for redox reactions. Areas of interest are the oxygen reduction reaction and the sweetening of gas streams by removal of hydrogen sulfide.
Phthalocyanine compounds have been investigated as donor materials in molecular electronics, e.g. organic field-effect transistors.[10]
Copper Phthalocyanine (CuPc) may possibly be used as storage in quantum computing, due to the length of time its electrons can remain in superposition.[11]
Toxicity and hazards
There is evidence that exposure to phthalocyanines can cause serious birth defects by copper depletion in developing embryos.[12]
Related compounds
Phthalocyanines are closely related to other tetrapyrrole macrocyles including porphyrins and porphyrazines. Structurally bigger analogues include single molecules like Naphthalocyanine and supramolecular complexes.
References
- ↑ Phthalocyanine at Sigma-Aldrich
- ↑ The Phthalocyanines, Vol. 1–4. C. C. Leznoff and A.B.P. Lever (eds.), Wiley 1986–1993.
- ↑ McKeown, Neil B. (1998) Phthalocyanine Materials – Synthesis, Structure and Function. Cambridge University Press
- ↑ The Porphyrin Handbook, Vols. 15–20; Karl Kadish, Kevin M. Smith, Roger Guilard (eds); Academic Press 2003.
- ↑ Iannuzzi, Marcella; Tran, Fabien; Widmer, Roland; Dienel, Thomas; Radican, Kevin; Ding, Yun; Hutter, Jürg; Gröning, Oliver (2014). "Site-selective adsorption of phthalocyanine on h-BN/Rh(111) nanomesh". Physical Chemistry Chemical Physics. 16 (24): 12374. doi:10.1039/C4CP01466A. PMID 24828002.
- ↑ Braun, A. & Tcherniac, J. (1907). "Über die Produkte der Einwirkung von Acetanhydrid auf Phthalamid" [On the products of the reaction of acetic anhydride with phthalamide]. Berichte der Deutschen Chemischen Gesellschaft. Deutsche Chemische Gesellschaft. 40 (2): 2709–2014. doi:10.1002/cber.190704002202.
- ↑ De Diesbach, Henri; von Der Weid, Edmond (1927). "Quelques sels complexes des o-dinitriles avec le cuivre et la pyridine". Helvetica Chimica Acta. 10: 886. doi:10.1002/hlca.192701001110.
- ↑ The Discovery of a New Pigment-The Story of Monastral Blue by Imperial Chemical Industries. colorantshistory.org
- 1 2 Gerd Löbbert "Phthalocyanines" in Ullmann's Encyclopedia of Industrial Chemistry, 2002, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a20_213.
- ↑ Chaidogiannos, G.; Petraki, F.; Glezos, N.; Kennou, S.; Nešpůrek, S. (2009). "Low voltage operating OFETs based on solution-processed metal phthalocyanines". Applied Physics A. 96 (3): 763. doi:10.1007/s00339-009-5268-1.
- ↑ New material for quantum computing discovered out of the blue. phys.org. October 27, 2013
- ↑ Sandor, S; Prelipceanu, O; Checiu, I (1985). "Sulphonated phthalocyanine induced caudal malformative syndrome in the chick embryo". Morphologie et embryologie. 31 (3): 173–81. PMID 2931590.
External links
| Wikimedia Commons has media related to Phthalocyanine. |
- Society of Porphyrins and Phthalocyanines
- Sir Patrick Linstead: Phthalocyanines (Video)
- Journal of Porphyrins and Phthalocyanines
- ICI GRANGEMOUTH DISCOVERY VIDEO
