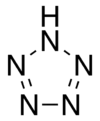
Pentazole
| |||
| Names | |||
|---|---|---|---|
| Systematic IUPAC name
1H-Pentazole[1] | |||
| Identifiers | |||
| 289-19-0 | |||
| 3D model (Jmol) | Interactive image Interactive image | ||
| ChemSpider | 4953932 | ||
| PubChem | 6451467 | ||
| |||
| |||
| Properties | |||
| N 5H | |||
| Molar mass | 71.0414 g/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Pentazole is an aromatic chemical molecule consisting of a five-membered ring with all nitrogen atoms, one of which is bonded to a hydrogen atom. It has a molecular formula HN5. Its SMILES structure is N1N[NH]NN1. Although strictly speaking a homocyclic, inorganic compound, pentazole has historically been classed as the last in a series of heterocyclic azole compounds containing one to five nitrogen atoms. This set contains pyrrole, imidazole, pyrazole, triazoles, tetrazoles, and pentazole.
Derivatives
Substituted analogs of pentazole are collectively known as pentazoles. As a class, they are unstable and often highly explosive compounds. The first pentazole synthesized was phenylpentazole, where the pentazole ring is highly stabilized by conjugation with the phenyl ring. The derivative 4-dimethylaminophenylpentazole is among the most stable pentazole compounds known, although it still decomposes at temperatures over 50 °C. It is known that electron-donating groups stabilize aryl pentazole compounds.[2]
Ions
The cyclic pentazolium cation (N+
5) is not known due to its probable antiaromatic character; whereas the open-chained pentazenium cation (N+
5) is known. Butler et al. first demonstrated the presence of the cyclic N−
5 in solution through the decomposition of substituted aryl pentazoles at low temperature. The presence of N
5H and N−
5 (held in solution through the interaction with zinc ions) was proven primarily using 15N NMR techniques of the decomposition products.[3][4] These results were initially challenged by some authors,[5] but subsequent experiments involving the detailed analysis of the decomposition products, complemented by computational studies, bore out the initial conclusion.[6][7][8] The pentazolide anion is not expected to last longer than a few seconds in aqueous solution without the aid of complexing agents. The discovery of pentazoles spurred attempts to create all-nitrogen salts such as N+
5N−
5, which should be highly potent propellants for space travel.
References
- ↑ "1H-Pentazole - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- ↑ Burke, L. A.; Fazen P. J. (Dec 2009). "Correlation Analysis of the Interconversion and Nitrogen Loss Reactions of Aryl Pentazenes and Pentazoles Derived From Aryl Diazonium and Azide Ions". International Journal of Quantum Chemistry. 109 (15): 3613–3618. Bibcode:2009IJQC..109.3613B. doi:10.1002/qua.22408.
- ↑ http://www.rsc.org/delivery/_ArticleLinking/DisplayHTMLArticleforfree.cfm?JournalCode=CC&Year=2003&ManuscriptID=b301491f&Iss=8
- ↑ http://www2.foi.se/rapp/foir1602.pdf
- ↑ Schroer T, HaigesR, Schneider S, Christe KO (2004-12-31). "The race for the first generation of the pentazolate anion in solution is far from over". Chemical Communications (12): 1607. doi:10.1039/B417010E.
- ↑ Butler RN, Hanniffy JM, Stephens JC, Burke LA (2008-01-31). "A Ceric Ammonium Nitrate N-Dearylation of N-p-Anisylazoles Applied to Pyrazole, Triazole, Tetrazole, and Pentazole Rings: Release of Parent Azoles. Generation of Unstable Pentazole, HN5/N5-, in Solution". The Journal of Organic Chemistry. 73 (4): 1354–1364. doi:10.1021/jo702423z. PMID 18198892.
- ↑ Perera SA, Gregusová A, Bartlett RJ (2009-04-01). "First Calculations of 15N−15N J Values and New Calculations of Chemical Shifts for High Nitrogen Systems: A Comment on the Long Search for HN5 and Its Pentazole Anion". The Journal of Physical Chemistry A. 113 (13): 3197–3201. doi:10.1021/jp809267y. PMID 19271757.
- ↑ http://www.irishtimes.com/news/science/galway-discovery-ahead-of-the-world-1.718468