Paramagnetic nuclear magnetic resonance spectroscopy
Paramagnetic NMR spectroscopy refers to NMR spectroscopy of paramagnetic compounds.[1][2] Although most NMR measurements are conducted on diamagnetic compounds, paramagnetic samples are also amenable to analysis and give rise to special effects indicated by a wide chemical shift range and broadened signals. Paramagnetism diminishes the resolution of an NMR spectrum to the extent that coupling is rarely resolved. Nonetheless spectra of paramagnetic provide insights into the bonding and structure of the sample. For example, the broadening of signals is compensated in part by the wide chemical shift range (often 200 ppm). Since paramagnetism leads to shorter relaxation times (1/T1), the rate of spectral acquisition can be high.
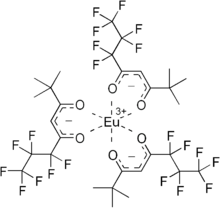 This europium complex is used as an "NMR shift reagent" because its presence shifts the NMR signals for many organic compounds.
This europium complex is used as an "NMR shift reagent" because its presence shifts the NMR signals for many organic compounds.
It should be noted that chemical shifts in diamagnetic compounds are described using the Ramsey equation, which describes so-called diamagnetic and paramagnetic contributions. In this equation, paramagnetic refers to excited state contributions, not to contributions from truly paramagnetic species.[1]
Isotropic shift
The difference between the chemical shift of a given nucleus in a diamagnetic vs paramagnetic environments is called the isotropic shift. The isotropic chemical shift for nickelocene is -255 ppm, which is the difference between the observed shift (ca. -260 ppm) and the shift observed for a diamagnetic analogue ferrocene (ca. 5 ppm). The isotropic shift contains contributions from the pseudocontact (called dipolar) and contact (also called scalar) terms.[3][4]
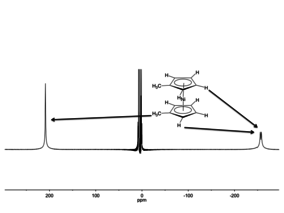
Contact vs pseudocontact shifts
Isotropic shifts result from two mechanisms, contact shifts and pseudocontact shifts. Both effects operate simultaneously but one or the other term can be dominant. Contact shifts result from spin polarization conveyed through the molecular orbitals of the molecule. Pseudocontact shifts result from the magnetic field emanating from the paramagnetic center. Pseudocontact shifts follow an 1/r3 dependence and tend to be smaller, often within the normal 1-10 ppm range for 1H NMR. NMR shift reagents such as EuFOD exploit this effect.[5]
The effect of the contact term arises from transfer of spin polarization to the observed nucleus. Spin polarization is a consequence of the very strong electron-nuclear (NMR detected) interaction. This coupling, also known by EPR spectroscopists as hyperfine coupling, is on the order of MHz, vs the usual internuclear (J) coupling observed in conventional NMR spectra, which are on the order of a few Hz. This difference reflects the large magnetic moment of an electron (−1.00 μB), which is much greater than any nuclear magnetic moment (e.g. for 1H: 1.52×10−3 μB). Owing to rapid spin relaxation, the electron-nuclear coupling is not observed in the NMR spectrum, so the affected nuclear resonance appears at the average of the two coupled energy states, weighted according to their spin populations. Given the magnitude of the coupling, the Boltzmann population of these spin state are not close to 1:1, leading to net spin polarization on the affected NMR nucleus, hence a relatively large contact shifts.[2]
The effect of the pseudocontact term arises magnetic anisotropy of the paramagnetic center (reflected in g-anisotropy in the EPR spectrum). This anisotropy creates a magnetic field which supplements that of the instrument's magnet. The magnetic field exerts its effect with both angular and a 1/r3 geometric dependences.
See also
- Electron paramagnetic resonance - a related technique for studying paramagnetic materials
References
- 1 2 Köhler, F. H., "Paramagnetic Complexes in Solution: The NMR Approach," in eMagRes, 2007, John Wiley. doi:10.1002/9780470034590.emrstm1229
- 1 2 R. S. Drago "Physical Methods in Inorganic Chemistry" 1977, W. B. Saunders, Philadelphia. ISBN 0-7216-3184-3
- ↑ Hrobárik, P., Reviakine, R., Arbuznikov, A. V., Malkina, O. L., Malkin, V. G., Köhler, F. H., Kaupp, M., "Density functional calculations of NMR shielding tensors for paramagnetic systems with arbitrary spin multiplicity: Validation on 3d metallocenes", J. Chem. Phys. 2007, volume 126, p. 024107. doi:10.1063/1.2423003
- ↑ Kruck, M., Sauer, D. C., Enders, M., Wadepohl, H., Gade, L. H., "Bis(2-pyridylimino)isoindolato iron(II) and cobalt(II) complexes: Structural chemistry and paramagnetic NMR spectroscopy", Dalton Trans. 2011, volume 40, p. 10406. doi:10.1039/c1dt10617a
- ↑ Friebolin, H., "Basic One- and Two- Dimensional NMR Spectroscopy," VCH: Weinheim, 2010. ISBN 978-3-527-32782-9.