Ophidiomyces ophiodiicola
Ophidiomyces ophiodiicola is a keratinophilic fungus from the family Onygenaceae of the order Onygenales. O. ophiodiicola is an emerging pathogen of captive and wild snakes in North America.[1] It is reported to cause snake fungal disease (SFD) in many different species of snakes; clinical signs include skin swelling, crusts, and nodules of the skin. The mode of transmission is unknown, but is speculated to occur with direct contact between snakes or with the contaminated environment. Currently no treatment for O. ophiodiicola is available. O. ophiodiicola was identified by Sigler, Hambleton & Paré in 2013. O. ophiodiicola is the only species in the genus Ophidiomyces. It was previously known as Chrysosporium ophiodiicola and is closely related to Chrysosporium anamorph Nannizziopsis vriesii (CANV).[1]
| Ophidiomyces ophiodiicola | |
|---|---|
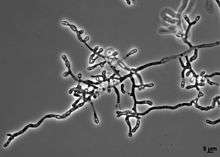 | |
| Hyphae of O. ophiodiicola | |
| Scientific classification | |
| Kingdom: | Fungi |
| Division: | Ascomycota |
| Subdivision: | Pezizomycotina |
| Class: | Eurotiomycetes |
| Order: | Onygenales |
| Genus: | Ophidiomyces |
| Species: | O. ophiodiicola |
| Binomial name | |
| Ophidiomyces ophiodiicola Sigler, Hambleton & Paré (2013) | |
| Synonyms | |
|
Chrysosporium ophiodiicola Guarro, Sutton, Wickes & Rajeev (2009) | |
Taxonomy and naming
Ophidiomyces ophiodiicolaa was first described as Chrysosporium ophiodiicola by Josef Guarro and colleagues in 2009 from infected snakes. Morphologically, the fungus resembled members of the genus Chrysosporium, and was though to be closely related to the reptile pathogen that had been referred to as the Chrysosporium anamorph Nannizziopsis vriesii (CANV). The genus Ophidiomyces was erected to accommodate this fungus in 2013 when DNA sequencing confirmed it to be a member of the famile Onygenaceae but genetically distinct from members of the genus Chrysosporium.[1]
Culture characteristics
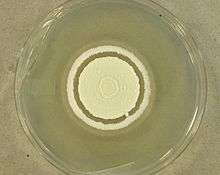
Cultures of O. ophiodiicola are powdery with whitish mycelium that becomes light yellowish with age. The cultures emit a pungent, skunk like odour. Optimal growth for O. ophiodiicola occurs at a temperature of 25 °C. Most isolates fail to grow at 35 °C. O. ophiodiicola is able to grow over pH range of 5-11 with optimal growth observed at pH of 9. O. ophiodiicola is able tolerate matric induced water stress below -5 MPa. The fungus exhibits strong urease activity and produces robust growth on ammonium sulfate, sulfite and thiosulfate.[2]
Morphology
No sexual state has been identified in the fungus O. ophiodiicola. Vegetative hyphae of O. ophiodiicola are narrow, branched and septate. Occasional racquet mycelia are observed. O. ophiodiicola reproduces asexually by the production of conidia. The conidia are produces at the end of short stalks ranging from 2.5 to 7.5 μm in length and 1.5 to 2.5 μm in width. The conidia range from 3 to 12.5 μm long and 1.3 to 3.5 μm wide.[3] and are released by rhexolytic dehiscence in which the walls of cell compartments adjacent to conidia erode, freeing the conidia from attached hyphae.[3] The conidia are colourless to pale yellow and smooth-walled.[2]
Ecology
Ecology of O. ophiodiicola is not well known but it is believed that O. ophiodiicola persist as an environmental saprobe in soil as well living hosts. O. ophiodiicola is able to utilize multiple carbon and nitrogen sources, tolerate range of pH, naturally occurring sulfur compound and low matric potential. These are most characteristics required to live in soil. Good growth on dead fish, insect, mushroom tissue and demineralized shrimp exoskeleton is observed. O. ophiodiicola physiological characteristics indicate that it is capable of growing in numerous ecosystem. [2]
Clinical symptoms
The mode of transmission is unknown, but is speculated to occur with direct contact between individuals or with the contaminated environment. Different symptoms can be seen in different species of snakes. In pit viper species facial swelling, cloudy eyes, improperly shed skin, roughened scales, dermal or subcutaneous granuloma and destruction of venom glands can be seen.[4] In massasaugas O. ophiodiicola infection infect deep muscle tissue and bone. Also lesions can be observed on the skin of the entire body.[2] In colubrids species snake fungal disease is reported to appear as pneumonia, ocular infection and subcutaneous nodules.[2] In Garter snake skin lesions are observed. The infection is reported to be systemic where it affects the lungs, liver and eyes.[2]
Pathogenicity in snakes

Infection begins in the outer most layer of the skin stratum corneum and progresses into the epidermis. Once infection reaches epidermis, snake's immune response gets activated and immune cells are recruited at the site of infection. Couple of days later, epidermis becomes necrotic and thickened.[5]
Lesions begins at the edge of individual scales and progress to adjacent scales. As lesions progress scales became rough and hyperpigmented. Lesions progressively become larger and severe until the snake shed its skin. Fluid filled vesicles formed between the new and old skin resulting in improper shedding of the skin; fragments of the old skin remains on the snake. Histological studies show skin lesions included areas of necrosis and granulocytic inflammation in the superficial to midepidermis.Mild chronic lymphoplasmacytic to lymphohistiocytic inflammation in the liver, lungs, heart, stomach and colon can be observed as well.[6]
There is a great conservation concern for snake population in the Eastern United States due SFD caused by O. ophiodiicola. Confirmed cases of SFD have been reported in nine states in USA though the disease is believed to be more widespread than has been documented. Multiple species of snakes that are affected including the Northern water snake (Nerodia sipedon), eastern racer (Coluber constrictor), rat snake (Pantherophis obsoletus species complex), timber rattlesnake (Crotalus horridus), massasauga (Sistrurus catenatus), pygmy rattlesnake (Sistrurus miliarius), and milk snake (Lampropeltis triangulum).[7] It is reported that population of rattlesnake in New Hampshire reduced to 19 from 40 due to SFD caused by O.ophiodiicola.[2]
There is no antifungal treatment available for O. ophiodiicola. Current option for managing SFD is rehabilitating individual snakes. Such a strategy is impractical for many snake populations because it can be difficult to locate the majority of individuals within the population, is resource intensive, and fails to prevent reinfection.[5] National Wildlife Health Center along with other organizations and researchers are working together to develop management strategies to mitigate disease impact.[7]
References
- 1 2 3 Rajeev, S.; Sutton, D. A.; Wickes, B. L.; Miller, D. L.; Giri, D.; Van Meter, M.; Thompson, E. H.; Rinaldi, M. G.; Romanelli, A. M.; Cano, J. F.; Guarro, J. (24 December 2008). "Isolation and Characterization of a New Fungal Species, Chrysosporium ophiodiicola, from a Mycotic Granuloma of a Black Rat Snake (Elaphe obsoleta obsoleta)". Journal of Clinical Microbiology. 47 (4): 1264–1268. doi:10.1128/JCM.01751-08.
- 1 2 3 4 5 6 7 Allender, Matthew C.; Raudabaugh, Daniel B.; Gleason, Frank H.; Miller, Andrew N. (October 2015). "The natural history, ecology, and epidemiology of Ophidiomyces ophiodiicola and its potential impact on free-ranging snake populations". Fungal Ecology. 17: 187–196. doi:10.1016/j.funeco.2015.05.003.
- 1 2 Sigler, L.; Hambleton, S.; Pare, J. A. (7 August 2013). "Molecular Characterization of Reptile Pathogens Currently Known as Members of the Chrysosporium Anamorph of Nannizziopsis vriesii Complex and Relationship with Some Human-Associated Isolates". Journal of Clinical Microbiology. 51 (10): 3338–3357. doi:10.1128/JCM.01465-13.
- ↑ Allender, M. C.; Bunick, D.; Dzhaman, E.; Burrus, L.; Maddox, C. (16 March 2015). "Development and use of a real-time polymerase chain reaction assay for the detection of Ophidiomyces ophiodiicola in snakes". Journal of Veterinary Diagnostic Investigation. 27 (2): 217–220. doi:10.1177/1040638715573983.
- 1 2 Lorch, Jeffrey M.; Knowles, Susan; Lankton, Julia S.; Michell, Kathy; Edwards, Jaime L.; Kapfer, Joshua M.; Staffen, Richard A.; Wild, Erik R.; Schmidt, Katie Z.; Ballmann, Anne E.; Blodgett, Doug; Farrell, Terence M.; Glorioso, Brad M.; Last, Lisa A.; Price, Steven J.; Schuler, Krysten L.; Smith, Christopher E.; Wellehan, James F. X.; Blehert, David S. (24 October 2016). "Snake fungal disease: an emerging threat to wild snakes". Philosophical Transactions of the Royal Society B: Biological Sciences. 371 (1709): 20150457. doi:10.1098/rstb.2015.0457.
- ↑ Lorch, J. M.; Lankton, J.; Werner, K.; Falendysz, E. A.; McCurley, K.; Blehert, D. S. (17 November 2015). "Experimental Infection of Snakes with Ophidiomyces ophiodiicola Causes Pathological Changes That Typify Snake Fungal Disease". mBio. 6 (6): e01534–15–e01534–15. doi:10.1128/mBio.01534-15.
- 1 2 "USGS National Wildlife Health Center - Snake Fungal Disease". www.nwhc.usgs.gov.