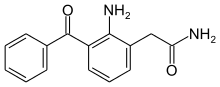
Nepafenac
 | |
| Clinical data | |
|---|---|
| Trade names | Nevanac |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a606007 |
| Pregnancy category |
|
| Routes of administration | Ophthalmic |
| ATC code | S01BC10 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| PubChem (CID) | 151075 |
| IUPHAR/BPS | 7564 |
| DrugBank |
DB06802 |
| ChemSpider |
133160 |
| UNII |
0J9L7J6V8C |
| KEGG |
D05143 |
| ChEBI |
CHEBI:75922 |
| ChEMBL |
CHEMBL1021 |
| ECHA InfoCard | 100.207.414 |
| Chemical and physical data | |
| Formula | C15H14N2O2 |
| Molar mass | 254.28 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Nepafenac is a non-steroidal anti-inflammatory drug (NSAID), usually sold as a prescription eye drop 0.1% solution. Nepafenac is manufactured by Alcon and sold under the trade name Nevanac. It is used to treat pain and inflammation associated with cataract surgery.[1]
The usual dose is one drop, thrice a day, in each affected eye beginning one day prior to cataract surgery, continued on the day of surgery and through the first two weeks of the postoperative period.[1]
Nepafenac is a prodrug of amfenac, an inhibitor of COX-1 and COX-2 activity.[2][3] Its side effects may include decreased visual acuity, a feeling that something is in the eye, increased eye pressure or a sticky sensation, as well as other effects.[1]
Brand names of Nepafenac include Ilevro and Nevanac.
References
- 1 2 3 Nepafenac Monograph
- ↑ Drugbank: Nepafenac
- ↑ Lira, R. P.; Fulco, E. A.; Chaves, A.; Da Costa Pinto, F.; Arieta, F. R.; Lira, C. E. (2012). "Effect of preoperative use of topical prednisolone acetate, ketorolac tromethamine, nepafenac and placebo, on the maintenance of intraoperative mydriasis during cataract surgery: A randomized trial". Indian Journal of Ophthalmology. 60 (4): 277–281. doi:10.4103/0301-4738.98705. PMC 3442462
 . PMID 22824596.
. PMID 22824596.