Levomefolic acid
 | |
| Names | |
|---|---|
| IUPAC name
(2S)-2-[ [4-[(2-Amino-5-methyl-4-oxo-1,6,7,8-tetrahydropteridin-6-yl) methylamino]benzoyl]amino]pentanedioic acid | |
| Other names
(L-5-Me-THFA, L-5-Me-H4FA), anion: L-5-methyltetrahydrofolate (L-5-Me-THF, L-5-Me-H4F), L-methylfolate Metafolin | |
| Identifiers | |
| 134-35-0 151533-22-1 (calcium salt) | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 392351 |
| KEGG | D09353 |
| MeSH | 5-methyltetrahydrofolate |
| PubChem | 444412 15341110 (calcium salt) |
| UNII | 8S95DH25XC |
| |
| |
| Properties | |
| C20H25N7O6 | |
| Molar mass | 459.46 g·mol−1 |
| Pharmacology | |
| B03BB51 (WHO) | |
| Legal status |
|
| |
| oral, transdermal, subcutaneous | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
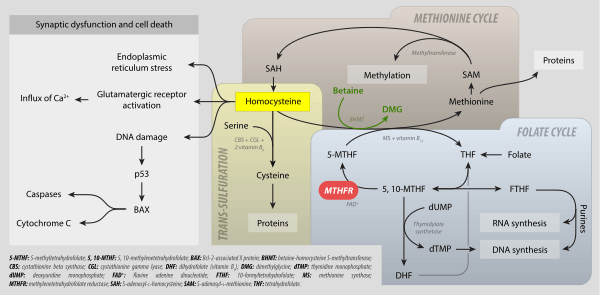
Levomefolic acid (INN) (also known as L-5-MTHF, L-methylfolate and L-5-methyltetrahydrofolate and (6S)-5-methyltetrahydrofolate, and (6S)-5-MTHF) is the primary biologically active form of folate used at the cellular level for DNA reproduction, the cysteine cycle and the regulation of homocysteine. It is also the form found in circulation and transported across membranes into tissues and across the blood-brain barrier. In the cell, L-methylfolate is used in the methylation of homocysteine to form methionine and tetrahydrofolate (THF). THF is the immediate acceptor of one carbon units for the synthesis of thymidine-DNA, purines (RNA and DNA) and methionine. The un-methylated form, folic acid (vitamin B9), is a synthetic form of folate, and must undergo enzymatic reduction by methylenetetrahydrofolate reductase (MTHFR) to become biologically active.[1]
It is synthesized in the absorptive cells of the small intestine from polyglutamylated dietary folate. It is a methylated derivative of tetrahydrofolate. Levomefolic acid is generated by MTHFR from 5,10-methylenetetrahydrofolate (MTHF) and used to recycle homocysteine back to methionine by methionine synthase (MS).[2]
L-methylfolate is water-soluble and primarily excreted via the kidneys. In a study of 21 subjects with coronary artery disease, peak plasma levels were reached in one to three hours following oral or parenteral administration. Peak concentrations were found to be more than seven times higher than folic acid (129 ng/ml vs. 14.1 ng/ml).[3]
Levomefolic acid (and folic acid in turn) has been proposed for treatment of cardiovascular disease[4][5] and advanced cancers such as breast and colorectal cancers.[6] It bypasses several metabolic steps in the body and better binds thymidylate synthase with FdUMP, a metabolite of the drug fluorouracil.
Levomefolate calcium, a calcium salt of levomefolic acid, developed and produced by Merck KGaA, is sold under the brand names Metafolin (a registered trademark of Merck KGaA) and incorporated in Deplin (trademark of Pamlab, LLC).[7]
Metabolism
Legal issues
In March 2012, Merck & Cie of Switzerland, Pamlab LLC (maker of Metanx and Cerefolin, Neevo DHA, and Deplin), and South Alabama Medical Science Foundation (SAMSF) (the plaintiffs) filed a complaint in the United States District Court for the Eastern District of Texas against four defendants - Macoven Pharmaceuticals (owned by Pernix Therapeutics), Gnosis SpA of Italy, Gnosis U.S.A and Gnosis Bioresearch Switzerland. The plaintiffs alleged that the defendants infringed on several of the plaintiffs' patents.[8] The Macoven products named in the suit are: "Vitaciric-B", "ALZ-NAC", "PNV DHA", and l-methylfolate calcium (levomefolate calcium).[9]
In September 2012, the same three plaintiffs filed a complaint requesting that the International Trade Commission begin an investigation of the same four defendants. The complaint states that Gnosis' "Extrafolic-S" and products which are made from it, infringe upon three of their patents: US 5997915, US 6673381, and US 7172778.[10]
See also
- 5,10-Methylenetetrahydrofolate (5,10-CH2-THF, MTHF)
- Methionine synthase (MS)
- Methylenetetrahydrofolate reductase (MTHFR)
- S-Adenosylmethionine (SAMe)
References
- ↑ Pietrzik, Klaus; Lynn Bailey; Barry Shane (2010). "Folic Acid and L-5-Methyltetrahydrofolate Comparison of Clinical Pharmacokinetics and Pharmacodynamics". Clinical Pharmacokinetics. 49 (8): 535–548. doi:10.2165/11532990-000000000-00000. PMID 20608755.
- ↑ "5-methyltetrahydrofolate - Compound Summary", PubChem, NCBI, retrieved 2012-09-25
- ↑ http://intetlab.com/site/products/Cerefolin-NAC_package-insert_%204-26-10.pdf
- ↑ Willems, Frank F; Boers GHJ; Blom HJ; Aengevaeren WRM; Verheugt FWA (March 2004). "Pharmacokinetic study on the utilisation of 5-methyltetrahydrofolate and folic acid in patients with coronary artery disease". Br J Pharmacol. Nature Publishing Group. 145 (5): 825–830. doi:10.1038/sj.bjp.0705446. PMC 1574248
 . PMID 14769778.
. PMID 14769778. - ↑ Iris P Fohr, Reinhild Prinz-Langenohl, Anja Brönstrup, Anja M Bohlmann, Heinz Nau, Heiner K Berthold, and Klaus Pietrzik, IP; Prinz-Langenohl, R; Brönstrup, A; Bohlmann, AM; Nau, H; Berthold, HK; Pietrzik, K (2002). "5,10-Methylenetetrahydrofolate reductase genotype determines the plasma homocysteine-lowering effect of supplementation with 5-methyltetrahydrofolate or folic acid in healthy young women" (PDF). Am J Clin Nutr. American Society for Clinical Nutrition. 75 (2): 275–282. PMID 11815318.
- ↑ Ströhle, Alexander; Wolters, Maike; Hahn, Andreas (2005). "Folic acid and colorectal cancer prevention: Molecular mechanisms and epidemiological evidence (Review)". International Journal of Oncology. doi:10.3892/ijo.26.6.1449. ISSN 1019-6439.
- ↑ Lakely, Susan. "RPH". L-Methylfolate. Retrieved 12 January 2012.
- ↑ The six patents named were US 5997915, US 6011040, US 6254904, US 6673381, US 7674490 and US 7172778.
- ↑ "Pernix Therapeutics' Subsidiary Macoven Pharmaceuticals, LLC Named in Suit by Merck, Pamlab, L.L.C. and Others for Alleged". Bloomberg. 6 March 2012. Retrieved 1 October 2012.
- ↑ Schweibenz, Eric W. (2012-09-10). "SAMSF, Merck, and Pamlab File New 337 Complaint Regarding Certain Reduced Folate Nutraceutical Products and L-methylfolate Raw Ingredients Used Therein". Oblon, Spivak, McClelland, Maier & Neustadt, L.L.P. Archived from the original on 1 October 2012.
External links
- CALCIUM L-5-METHYLTETRAHYDROFOLATE (L-5-MTHF-Ca)
- Brockton, N. T. (2006). "Localized depletion: The key to colorectal cancer risk mediated by MTHFR genotype and folate?". Cancer Causes & Control. 17 (8): 1005–16. doi:10.1007/s10552-006-0051-5. PMID 16933051.
- Stahl, SM (October 2007). "Novel Therapeutics for Depression: L-methylfolate as a Trimonoamine Modulator and Antidepressant-Augmenting Agent". CNS Spectrums. 12 (10): 739–744. PMID 17934378.