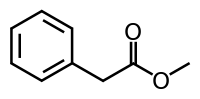
Methyl phenylacetate
 | |
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Methyl phenylacetate | |
| Other names
Methyl 2-phenylacetate Methyl benzene acetate | |
| Identifiers | |
| 101-41-7 | |
| 3D model (Jmol) | Interactive image |
| 878795 | |
| ChemSpider | 7278 |
| ECHA InfoCard | 100.002.674 |
| EC Number | 202-940-9 |
| MeSH | C024906 |
| PubChem | 7559 |
| UNII | D4PDC41X96 |
| |
| |
| Properties | |
| C9H10O2 | |
| Molar mass | 150.1745 g mol−1 |
| Appearance | Colorless liquid |
| Density | 1.055 g/cm3 (± 0.06) |
| Melting point | 50 °C (122 °F; 323 K) |
| Boiling point | 218 °C (424 °F; 491 K) |
| 2070 mg/L | |
| Vapor pressure | 17.3 Pa |
| Refractive index (nD) |
1.505 (± 0.02) at 20 °C |
| Hazards | |
| NFPA 704 | |
| Flash point | 90.6 °C (195.1 °F; 363.8 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Methyl phenylacetate is an organic compound that is the ester formed from methanol and phenylacetic acid, with the structural formula C6H5CH2COOCH3. It is a clear colorless liquid that is only slightly soluble in water, but very soluble in most organic solvents.
Methyl phenylacetate has a strong odor similar to honey. The odor is so strong that recommended smelling is of a solution with 10% or less methyl phenylacetate. This compound also naturally occurs in brandy, capsicum, coffee, honey, pepper and some wine.
Methyl phenylacetate is used in the flavor industry and in perfumes to impart honey scents.
References
- "Methyl Phenyl Acetate."(February 22, 2007). Chemical Information The Good Scents Company. Retrieved on January 22, 2008.
This article is issued from Wikipedia - version of the 9/28/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.
