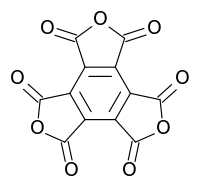
Mellitic anhydride
 | |
 | |
| Names | |
|---|---|
| IUPAC name
4,9,14-trioxatetracyclo[10.3.0.02,6.07,11]pentadeca-1,6,11-triene-3,5,8,10,13,15-hexone | |
| Identifiers | |
| 4253-24-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 223826 |
| PubChem | 255291 |
| |
| |
| Properties | |
| C12O9 | |
| Molar mass | 288.12 g·mol−1 |
| Appearance | colorless solid[1] |
| Melting point | 161 °C; 322 °F; 434 K [1] |
| Vapor pressure | 0.000004 mmHg (20°C)[1] |
| Hazards | |
| US health exposure limits (NIOSH): | |
| PEL (Permissible) |
none[1] |
| REL (Recommended) |
TWA 0.005 ppm (0.04 mg/m3) Should be handled in the workplace as an extremely toxic substance.[1] |
| IDLH (Immediate danger) |
N.D.[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Mellitic anhydride, the anhydride of mellitic acid, is an organic compound with the formula C12O9.
Mellitic anhydride is an oxide of carbon (oxocarbon), like CO2, CO, and C3O2. It is a white sublimable solid, apparently obtained by Liebig and Wöhler in 1830 in their study of mellite ("honey stone"); they assigned it the formula C4O3.[2][3][4] The substance was properly characterized in 1913 by H. Meyer and K. Steiner.[5][6] It retains the aromatic character of the benzene ring.[7][8]
References
- 1 2 3 4 5 6 "NIOSH Pocket Guide to Chemical Hazards #0635". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Wöhler, F. (1826). "Ueber die Honigsteinsäure". Annalen der Physik und Chemie. 83 (7): 325–334. doi:10.1002/andp.18260830706.
- ↑ Liebig, J.; Wöhler, F. (1830). "Ueber die Zusammensetzung der Honigsteinsäure". Annalen der Physik und Chemie. 94 (2): 161–164. doi:10.1002/andp.18300940202.
- ↑ Erdmann, O. L.; Marchand, R. F. (1848). "Ueber die Mellithsäure". Journal für Praktische Chemie. 43 (2/3): 129–144. doi:10.1002/prac.18480430113.
- ↑ Meyer, H.; Steiner, K. (1913). "Über ein neues Kohlenoxyd C12O9" [A new carbon oxide C12O9]. Berichte der Deutschen Chemischen Gesellschaft. 46 (1): 813–815. doi:10.1002/cber.191304601105.
- ↑ Bugge, G. (1914). "Chemie: Ein neues Kohlenoxyd". Naturwissenschaftliche Wochenschrift. 13/29 (12): 188.
- ↑ Fowler, P. W.; Lillington, M. (2007). "Mellitic Trianhydride, C12O9: The Aromatic Oxide of Carbon". Journal of Chemical Information and Modeling. 47 (3): 905–908. doi:10.1021/ci600547n.
- ↑ Ermer, O.; Neudörfl, J. (2000). "Structure of Mellitic Trianhydride". Helvetica Chimica Acta. 83 (1): 300–309. doi:10.1002/(SICI)1522-2675(20000119)83:1<300::AID-HLCA300>3.0.CO;2-L.
This article is issued from Wikipedia - version of the 9/22/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.