Maribavir
 | |
| Clinical data | |
|---|---|
| ATC code | J05AX10 (WHO) |
| Identifiers | |
| |
| CAS Number |
176161-24-3 |
| PubChem (CID) | 471161 |
| ChemSpider |
413807 |
| UNII |
PTB4X93HE1 |
| NIAID ChemDB | 070966 |
| Chemical and physical data | |
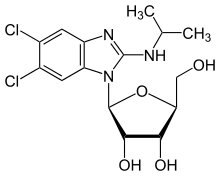
| Formula | C15H19Cl2N3O4 |
| Molar mass | 376.24 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Maribavir (originally named 1263W94) is an experimental oral antiviral drug candidate licensed by ViroPharma from GlaxoSmithKline in 2003 for the prevention and treatment of human cytomegalovirus (HCMV) disease in hematopoietic stem cell/bone marrow transplant patients. The mechanism by which maribavir inhibits HCMV replication is by inhibition of an HCMV encoded protein kinase enzyme called UL97 or pUL97.[1] Maribavir showed promise in Phase II clinical trials and was granted fast track status, but failed to meet study goals in a Phase III trial.[2] However, the dosage used in the Phase III trial may have been too low to be efficacious.[3]
A Phase II study with maribavir demonstrated that prophylaxis with maribavir displayed strong antiviral activity, as measured by statistically significant reduction in the rate of reactivation of CMV in recipients of hematopoietic stem cell/bone marrow transplants.[4] In an intent-to-treat analysis of the first 100 days after the transplant, the number of subjects who required pre-emptive anti-CMV therapy was statistically significantly reduced with maribavir compared to placebo.
ViroPharma conducted a Phase III clinical study to evaluate the prophylactic use for the prevention of cytomegalovirus disease in recipients of allogeneic stem cell transplant patients. In February 2009, ViroPharma announced that the Phase III study failed to achieve its goal, showing no significant difference between maribavir and a placebo at reducing the rate at which CMV DNA levels were detected in patients.[5]
References
- ↑ Potent and Selective Inhibition of Human Cytomegalovirus Replication by 1263W94, a Benzimidazole L-Riboside with a Unique Mode of ActionAntimicrobial Agents and Chemotherapy (2002) Vol 46 (8): 2365–2372
- ↑ Maribavir prophylaxis for prevention of cytomegalovirus disease in recipients of allogeneic stem-cell transplants: a phase 3, double-blind, placebo-controlled, randomised trial, The Lancet Infectious Diseases (2011) Vol 11 (4): 284–292
- ↑ Why did maribavir fail in stem-cell transplants?, The Lancet Infectious Diseases (2011) Vol 11 (4): 255–257
- ↑ Phase 2 Data Shows Maribavir Markedly Reduced Rate Of Cytomegalovirus Infection And Disease In Bone Marrow Transplant Patients, Medical News Today, Jun 2, 2008
- ↑ ViroPharma:Maribavir Phase III Study Missed Goal;Shares Plunge, CNN Money, February 09, 2009