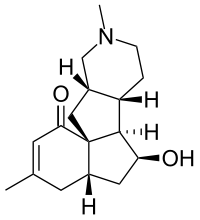
Magellanine
 | |
| Names | |
|---|---|
| IUPAC name
(4aS,6S,6aR,6bS,10aS,11aS)-6-Hydroxy-3,9-dimethyl-4,4a,5,6,6a,6b,7,8,9,10,10a,11-dodecahydro-1H-benzo[3a,4]pentaleno[2,1-c]pyridin-1-one | |
| Identifiers | |
| 61273-75-4 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 390919 |
| PubChem | 442489 |
| |
| |
| Properties | |
| C17H25NO2 | |
| Molar mass | 275.39 g·mol−1 |
| Melting point | 165 to 166 °C (329 to 331 °F; 438 to 439 K)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
(−)-Magellanine is a member of the Lycopodium alkaloid class of natural products. It was isolated from the club moss Lycopodium magellanicum in 1976.[1] It has been synthesized five times, with the first synthesis having been completed by the Larry E. Overman group at the University of California, Irvine in 1993.[2] It has also been synthesized by the Leo Paquette group in 1993 at The Ohio State University,[3] the Chun-Chen Liao group in 2002 at National Tsing Hua University,[4] the Miyuki Ishikazi and Tamiko Takahashi groups in 2005 at the Josai International University and Tokyo University of Science,[5] and the Chisato Mukai group in 2007 at the Kanazawa University.[6] One partial synthesis was completed by the A. I. Meyers group in 1995 at Colorado State University. [7]
Biosynthetically, it is thought to have been derived from lysine. This was determined by conducting feeding studies of radiolabeled precursors. [8]
References
- 1 2 Isolation and Structure Canadian Journal of Chemistry, 1976, 54:(18) 2893-2899.
- ↑ Larry E. Overman Synthesis. J. Am. Chem. Soc., 1993, 115 (7), pp 2992–2993.
- ↑ Leo Paquette Synthesis J. Am. Chem. Soc., 1994, 116 (11), pp 4689–4696.
- ↑ Chun-Chen Liao Synthesis Angew. Chem. Int. Ed. 2002, 41, No. 21, 4090-4093.
- ↑ Miyuki Ishikazi and Yamiko Takahashi Synthesis Tetrahedron 61 (2005) 4053–4065.
- ↑ Chisato Mukai Synthesis J. Org. Chem. 2007, 26, 10147-10154.
- ↑ A. I. Meyers Partial Synthesis J. Chem. Soc., Chem. Commun., 1995, 2511-2512.
- ↑ Biosynthesis of the Lycopodium Alkaloids Nat. Prod. Rep., 2004, 21, 752-772.