Macrocycle
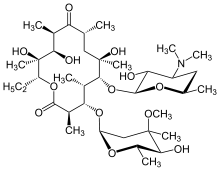
A macrocycle is, as defined by IUPAC, "a cyclic macromolecule or a macromolecular cyclic portion of a molecule".[1] In the chemical literature, macrocycles varyingly include molecules containing rings of 8 or more atoms,[2][3] or 12 or more atoms.[4] Well-known examples are the group of drugs known as macrolides. The IUPAC definition notes that a "cyclic macromolecule has no end-groups but may nevertheless be regarded as a chain", and that "macrocycle is sometimes used [in the literature] for molecules of low relative molecular mass that are not considered macromolecules.[5] Many macrocycles are employed as macrocyclic ligands.
Synthesis
In general, macrocycles are synthesized from smaller, usually linear, molecules.
There are two major categories for synthesizing macrocycles, priority in both being to maximize yields of the required product by choosing strategies which inhibit competing linear polymerization and other reactions.[6]
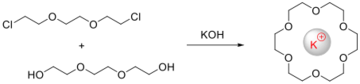
Direct synthesis
This cyclization proceeds by a conventional organic reaction, not requiring any kind of directional influence of a metal ion. Usually involves equimolar concentration of the reagents in order to produce a 1:1 condensation reaction, either at high-dilution conditions or moderate to low dilution conditions.
Template synthesis
Some cyclizations are facilitated by the presence of metal ions, which helps organize the formation of the macrocyclic ligand. The metal ion acts as a 'template' for the cyclization reaction. Phthalocyanine was the first synthetic macrocycle produced by template reaction. The metal ion may direct the condensation preferentially to cyclic rather than polymeric products (the kinetic template effect) or stabilize the macrocycle once formed (the thermodynamic template effect).
Related molecular categories
- Cryptand: a macrocycle with multiple loops (e.g., bicyclic).
- Rotaxane: macrocycle(s) stuck on a stick, generally freely
- Catenane: interlocked molecular rings (like a chain).
- Molecular knot: a molecule in the shape of a knot such as a trefoil knot.
See also
References
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "macrocycle".
- ↑ Still, W. C.; Galynker, I. Tetrahedron 1981, 37, 3981-3996.
- ↑ J. D. Dunitz. Perspectives in Structural Chemistry (Edited by J. D. Dunitz and J. A. Ibers), Vol. 2, pp. l-70; Wiley, New York (1968)
- ↑ Parker, S.P. (2002). McGraw-Hill Science & Technology Dictionary. McGraw-Hill. ISBN 978-0070423138.
- ↑ "Glossary of basic terms in polymer science (IUPAC Recommendations 1996)" (PDF). Pure and Applied Chemistry. 68 (12): 2287–2311. 1996. doi:10.1351/pac199668122287.
- ↑ L.F Lindloy, The Chemistry of Macrocylic Ligand Complexes, Cambridge University Press, 1989, 20-50 ISBN 0-521-25261-X
Further reading
- Chambron, J-C.; Dietrich-Buchecker, C.; Hemmert, C.; Khemiss, A-K.; Mitchell, D.; Sauvage, J-P.; Weiss, J. (1990). "Interlacing molecular threads on transition metals" (PDF). Pure Appl. Chem. 62 (6): 1027–34. doi:10.1351/pac199062061027.